Reaction: ST8SIA2,3,6 transfer Neu5Ac to terminal Gal of N-glycans
- in pathway: N-Glycan antennae elongation
Addition of sialic acid (Neu5Ac) to galactose-containing N-glycan. Sialic acid is usually found at terminal positions of the N-glycan. This imparts a negative charge at neutral pH which affects the chemico-physical and biological properties of the N-glycans (for a review, see Schauer 2000); moreover, this modification can lead to the addition of extraordinarily long antennae such as polysialic acid (hundreds of sials) or polylactosamine repeats (dozens of disaccharide repeats) (Harduin-Lepers 2001), while the number of modifications on the antennae of N-glycans is usually lower.
There are over 20 sialyltransferases known in humans, 5 of which are known to act on N-glycans. Beta-galactoside alpha-2,6-sialyltransferase 1
(ST6GAL1) is the only sialyltransferase known to transfer Neu5Ac to Gal on N-Glycans (Dall'Olio 2000). A second beta-galactoside alpha-2,6-sialyltransferase has been characterized, but this enzyme acts mainly on oligosaccharides (Krzewinski-Recchi et al. 2003). Neu5Ac can also be added via an alpha-2,3-linkage to Gal on N-glycans by CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,3-sialyltransferase 4 (ST3GAL4) (Ellies et al. 2002). ST8Sia II (ST8SIA2), ST8Sia III (ST8SIA3), and ST8Sia IV (ST8SIA6) have alpha-2,8-activity (Angata et al. 1997, Angata et al. 2000, Angata & Fuduka 2003).
There are over 20 sialyltransferases known in humans, 5 of which are known to act on N-glycans. Beta-galactoside alpha-2,6-sialyltransferase 1
(ST6GAL1) is the only sialyltransferase known to transfer Neu5Ac to Gal on N-Glycans (Dall'Olio 2000). A second beta-galactoside alpha-2,6-sialyltransferase has been characterized, but this enzyme acts mainly on oligosaccharides (Krzewinski-Recchi et al. 2003). Neu5Ac can also be added via an alpha-2,3-linkage to Gal on N-glycans by CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,3-sialyltransferase 4 (ST3GAL4) (Ellies et al. 2002). ST8Sia II (ST8SIA2), ST8Sia III (ST8SIA3), and ST8Sia IV (ST8SIA6) have alpha-2,8-activity (Angata et al. 1997, Angata et al. 2000, Angata & Fuduka 2003).
Reaction - small molecule participants:
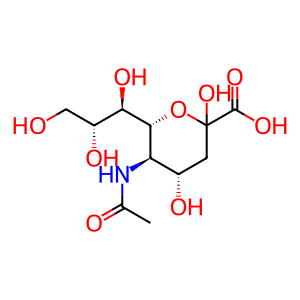
Neu5Ac [Golgi lumen]
NGP [Golgi lumen]
Reactome.org reaction link: R-HSA-1022133
======
Reaction input - small molecules:
N-acetylneuraminic acid
N-glycan
Reaction output - small molecules:
Reactome.org link: R-HSA-1022133