Reaction: Trimming of peptides by IRAP in endocytic vesicles
- in pathway: Endosomal/Vacuolar pathway
While it is established that cathepsin S is involved in antigen processing in endocytotic vesicles, it is less certain whether other proteases present in endocytic vesicles are also involved in generating the peptide fragments. Insulin regulated aminopeptidase (IRAP) is an epitope-trimming zinc-dependent aminopeptidase closely related to ERAP1 and ERAP2. IRAP may be involved in vacuolar processing of the peptide fragments within endosomes (Saveanu et al. 2009, Segura et al. 2009). IRAP is detected predominantly in the early and recycling endosome fractions. Saveanu et al. (2009) observed the physical association of IRAP with internalized class I MHC molecules and suggested that this may favour a direct linkage between peptide trimming and MHC class I loading. They also showed that IRAP-dependent processing of antigens requires active proteasome but not lysosomal proteases, which suggests that this pathway utilizes cytosolic degradation followed by peptide transport into IRAP-containing endosomes.
Reaction - small molecule participants:
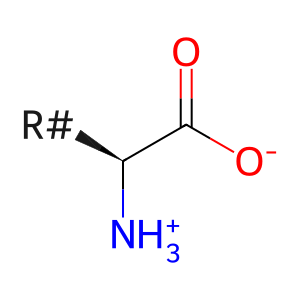
L-amino acid [early endosome lumen]
Antigen peptide [early endosome lumen]
precursor peptide fragment (>16 aa) [early endosome lumen]
Reactome.org reaction link: R-HSA-1236954
======
Reaction input - small molecules:
oligopeptide
Reaction output - small molecules:
L-alpha-amino acid zwitterion
oligopeptide
Reactome.org link: R-HSA-1236954