Reaction: SMOX-3 oxidises SPN to SPM
- in pathway: Interconversion of polyamines
Spermine oxidase (SMOX, PAOh1, SMO) is a polyamine oxidase flavoenzyme that catalyses the oxidation of spermine (SPN) to spermidine (SPM). It plays an important role in the regulation of endogenous polyamine intracellular concentration. Five different isozymes are produced by alternative splicing with isozyme 3 being the major isoform and possessing the highest affinity for spermine. It is highly inducible by specific antitumor polyamine analogues (Wang et al. 2001).
Reaction - small molecule participants:
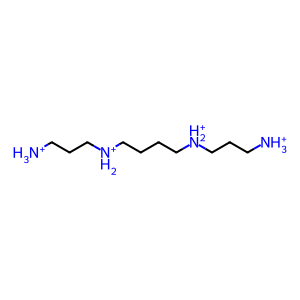
SPM [peroxisomal matrix]
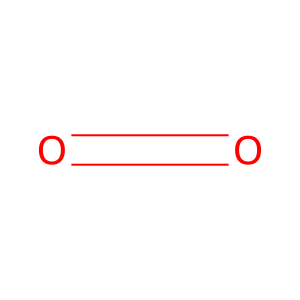
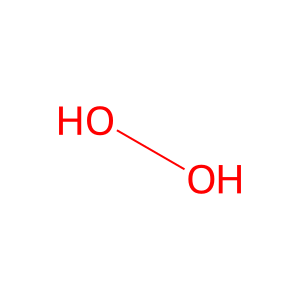
H2O2 [peroxisomal matrix]
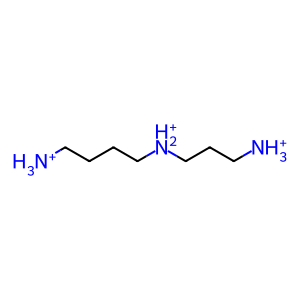
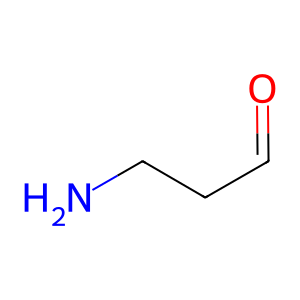
3APAL [peroxisomal matrix]
O2 [peroxisomal matrix]
H2O [peroxisomal matrix]
SPN [peroxisomal matrix]
SPM [peroxisomal matrix]
H2O2 [peroxisomal matrix]
3APAL [peroxisomal matrix]
O2 [peroxisomal matrix]
H2O [peroxisomal matrix]
SPN [peroxisomal matrix]
Reactome.org reaction link: R-HSA-141341
======
Reaction input - small molecules:
dioxygen
water
spermine(4+)
dioxygen
water
spermine(4+)
Reaction output - small molecules:
spermidine(3+)
hydrogen peroxide
3-aminopropanal
spermidine(3+)
hydrogen peroxide
3-aminopropanal
Reactome.org link: R-HSA-141341