Reaction: Phosphorylation and activation of VAV1
- in pathway: Signaling by SCF-KIT
The Src and PI3-kinase signaling pathways converge to activate Rac1 and JNK after c-Kit activation, promoting mast cell proliferation but not for suppression of apoptosis (Timokhina et al. 1998). PI3K and Src are considered mediators of c-Kit induced Rac1 activation via the guanine nucleotide exchange factor (GEF) VAV1. Stimulation of c-Kit receptor results in rapid tyrosine phosphorylation of VAV1 (Timokhina et al. 1998).
VAV1 exists in an auto-inhibitory state folded in such a way as to inhibit the GEF activity of its Dbl homology domain (DH) domain. PI3K is thought to modulate the activation of VAV1 by influencing its degree of tyrosine phosphorylation and its recruitment to membrane. VAV1 is recruited to membrane by binding to PtdIns(3,4,5)P3 (PIP3) and this interaction relieves an intramolecular interaction between pleckstrin homology (PH) and DH domains, thus facilitating tyrosine phosphorylation on Y174 and so further opening of the DH/PH domains, binding of Rac-GDP and catalysis (Welch et al, 2003). In VAV1, tyrosine 174 (Y174) binds to the DH domain and inhibits its GEF activity. Src kinases phosphorylate this Y174 and this causes the tyrosine to move away from the DH domain thereby reliving the auto-inhibition.
VAV1 exists in an auto-inhibitory state folded in such a way as to inhibit the GEF activity of its Dbl homology domain (DH) domain. PI3K is thought to modulate the activation of VAV1 by influencing its degree of tyrosine phosphorylation and its recruitment to membrane. VAV1 is recruited to membrane by binding to PtdIns(3,4,5)P3 (PIP3) and this interaction relieves an intramolecular interaction between pleckstrin homology (PH) and DH domains, thus facilitating tyrosine phosphorylation on Y174 and so further opening of the DH/PH domains, binding of Rac-GDP and catalysis (Welch et al, 2003). In VAV1, tyrosine 174 (Y174) binds to the DH domain and inhibits its GEF activity. Src kinases phosphorylate this Y174 and this causes the tyrosine to move away from the DH domain thereby reliving the auto-inhibition.
Reaction - small molecule participants:
ADP [cytosol]
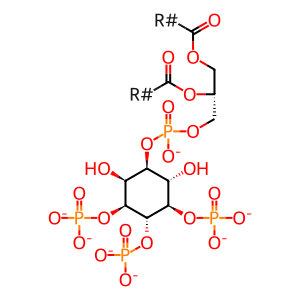
PI(3,4,5)P3 [plasma membrane]
ATP [cytosol]
Reactome.org reaction link: R-HSA-1433542
======
Reaction input - small molecules:
1-phosphatidyl-1D-myo-inositol 3,4,5-trisphosphate(7-)
ATP(4-)
Reaction output - small molecules:
ADP(3-)
Reactome.org link: R-HSA-1433542