Reaction: bHEXA hydrolyzes GM2A:GM2 to GM2A:GM3
- in pathway: Glycosphingolipid catabolism
Beta-hexosaminidase A complex (bHEXA) cleaves the terminal N-acetyl galactosamine from GM2 ganglioside to form GM3 ganglioside (Lemieux et al. 2006). There are two forms of hexosaminidase complexes: hexosaminidase A and B. The A form is a trimer of the subunits alpha (HEXA, beta A) and beta (HEXB, beta B). The B form is a tetramer of 2 alpha and two beta subunits (O'Dowd et al. 1988). Only form A is active toward GM2 ganglioside (Conzelmann & Sandhoff 1979). GM2 activator (GM2A, GM2AP) acts as an essential cofactor to the reaction by mobilizing GM2 from intralysosomal vehicle (ILV) membranes and binding to bHEXA (Kytzia & Sandhoff, 1985; Yadao et al., 1997; Ravasi et al., 2002; Wendeler et al., 2006). Defects in the two complex subunits or GM2A cause lysosomal storage diseases marked by the accumulation of GM2 ganglioside in neuronal cells. Defects in the alpha subunits are the cause of GM2-gangliosidosis type 1 (GM2G1) (MIM:272800), also known as Tay-Sachs disease (Nakano et al. 1988). Defects in the beta subunits are the cause of GM2-gangliosidosis type 2 (GM2G2) (MIM:268800), also known as Sandhoff disease (Banerjee et al. 1991). Defects in GM2A function lead to GM2 gangliosidosis AB (MIM:272750), also known as the Tay-Sachs disease AB variant (Schroeder et al., 1991; Wilkening et al., 2000; reviewed by Sandhoff & Sandhoff, 2018).
Reaction - small molecule participants:
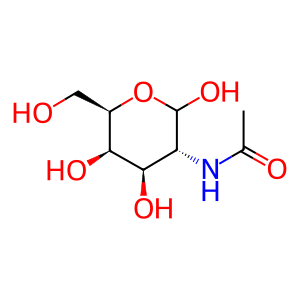
GalNAc [lysosomal lumen]
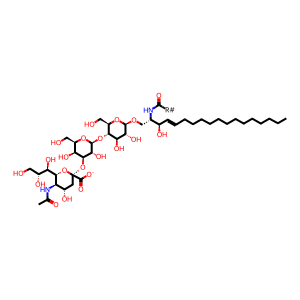
GM3 [lysosomal lumen]
H2O [lysosomal lumen]
Reactome.org reaction link: R-HSA-1605595
======
Reaction input - small molecules:
water
Reaction output - small molecules:
N-acetyl-D-galactosamine
alpha-N-acetylneuraminosyl-(2->3)-beta-D-galactosyl-(1->4)-beta-D-glucosyl-(1<->1')-N-acylsphingosine(1-)
Reactome.org link: R-HSA-1605595