Reaction: Fucosylation of Pre-NOTCH by POFUT1
- in pathway: Pre-NOTCH Processing in the Endoplasmic Reticulum
In the endoplasmic reticulum, NOTCH receptor precursors are fucosylated on conserved serine and threonine residues in their EGF repeats. The consensus fucosylation site sequence is C2-X(4-5)-S/T-C3, where C2 and C3 are the second and third cysteine residue within the EGF repeat, and X(4-5) is four to five amino acid residues of any type. Only those fucosylation sites that are conserved between human, mouse and rat NOTCH isoforms are annotated. Two additional potential fucosylation sites exist in human NOTCH1, on threonine 194 and threonine 1321, but since they are not conserved between all three species, they are not shown. Fucosylation is performed by the endoplasmic reticulum resident O-fucosyl transferase (POFUT1). Fucosylation by POFUT1 is considered to be essential for NOTCH folding/processing and production of a fully functional receptor. In addition to Notch fucosylation, Drosophila Pofut1 (o-fut1) acts as a Notch chaperone, playing an important role in Notch trafficking (Okajima et al. 2005). The chaperone role of POFUT1 may not be conserved in mammals (Stahl et al. 2008).
Reaction - small molecule participants:
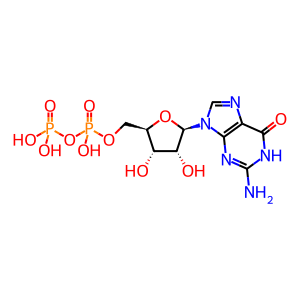
GDP [endoplasmic reticulum lumen]
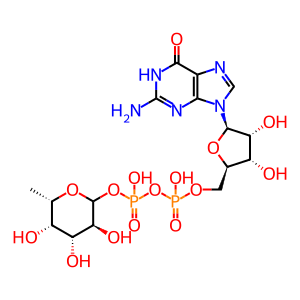
GDP-Fuc [endoplasmic reticulum lumen]
Reactome.org reaction link: R-HSA-1912349
======
Reaction input - small molecules:
GDP-L-fucose
Reaction output - small molecules:
GDP
Reactome.org link: R-HSA-1912349