Reaction: PDP dephosphorylates p-lipo-PDH
- in pathway: Regulation of pyruvate dehydrogenase (PDH) complex
Pyruvate dehydrogenase phosphatase (PDP) in the mitochondrial matrix catalyzes the hydrolytic removal of phosphate groups from phosphoserine residues in the E1 alpha subunit of the pyruvate dehydrogenase complex. The active form of PDP is a heterodimer of a catalytic subunit and a regulatory one. Two isoforms of the catalytic subunit have been identified and biochemically characterized (Huang et al. 1998) and mutations in PDP1 have been associated with PDP deficiency in vivo (Maj et al. 2005). The properties of the human regulatory subunit have been deduced from those of its bovine homologue (Lawson et al. 1997). The activity of PDP1 is greatly enhanced through Ca2+ -dependent binding of the catalytic subunit (PDP1c) to the L2 (inner lipoyl) domain of dihydrolipoyl acetyltransferase (E2), which is also integrated in the pyruvate dehydrogenase complex. PDP activity requires Mg2+ (Huang et al. 1998).
Reaction - small molecule participants:
Pi [mitochondrial matrix]
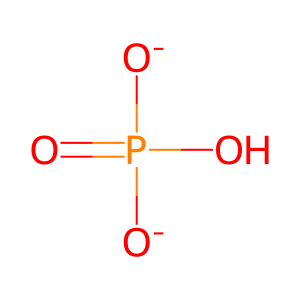
H2O [mitochondrial matrix]
Reactome.org reaction link: R-HSA-204169
======
Reaction input - small molecules:
water
Reaction output - small molecules:
hydrogenphosphate
Reactome.org link: R-HSA-204169