Reaction: Phosphorylation of beta and gamma subunits by LYN
- in pathway: Fc epsilon receptor (FCERI) signaling
Upon FCGRI-IgE aggregation, LYN kinase phosphorylates the tyrosine residues within the ITAM (immunoreceptor tyrosine-based activation motifs) of both the beta and gamma subunits. The detailed mechanism of the initial engagement of LYN kinase and FCERI is incompletely understood, but two different models have been proposed. One model postulates that a small fraction of LYN is constitutively bound to beta subunit of FCERI prior to activation. Aggregation of FCERI facilitates the transphosphorylation of one FCERI by LYN bound to a juxtaposed receptor (Vonakis et al. 1997, Draber & Draberova 2002). Alternative model postulates that LYN is observed in lipid rafts enriched in glycosphingolipids, cholesterol, and glycosylphosphatidylinositol-anchored proteins and upon aggregation, FCERI rapidly translocates into lipid rafts, where it is phosphorylated by LYN kinase. Either the association of LYN or FCERI or both with lipid rafts is important for initiating this phosphorylation process (Young et al. 2003, Kovarova et al. 2002, Draber & Draberova 2002).
Beta subunit ITAM differs from canonical ITAMs in two ways; the spacing between the two canonical tyrosines harbours a third tyrosine, and it is one amino acid shorter than in canonical ITAMs, thus making it unfit to bind and recruit Syk. Among the three tyrosine residues (Y219, Y225 and Y229), Y219 may play a predominant role in beta chain function and LYN recruitment. Mutation of this tyrosine would decrease substantially LYN association and subsequent phosphorylation of Y225 and Y229. This would result in decreased gamma phosphorylation and decreased SYK recruitment and activation (On et al. 2004).
Beta subunit ITAM differs from canonical ITAMs in two ways; the spacing between the two canonical tyrosines harbours a third tyrosine, and it is one amino acid shorter than in canonical ITAMs, thus making it unfit to bind and recruit Syk. Among the three tyrosine residues (Y219, Y225 and Y229), Y219 may play a predominant role in beta chain function and LYN recruitment. Mutation of this tyrosine would decrease substantially LYN association and subsequent phosphorylation of Y225 and Y229. This would result in decreased gamma phosphorylation and decreased SYK recruitment and activation (On et al. 2004).
Reaction - small molecule participants:
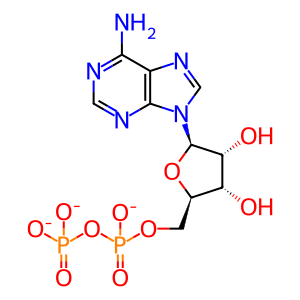
ADP [cytosol]
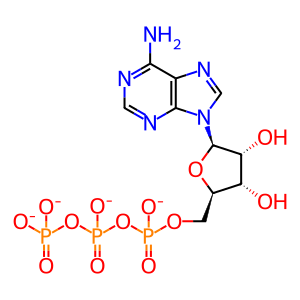
ATP [cytosol]
Reactome.org reaction link: R-HSA-2454208
======
Reaction input - small molecules:
ATP(4-)
Reaction output - small molecules:
ADP(3-)
Reactome.org link: R-HSA-2454208