Reaction: Acetylation of SMC3 subunit of chromosomal arm associated cohesin by ESCO1 or ESCO2
- in pathway: Establishment of Sister Chromatid Cohesion
Acetyltransferases ESCO1 and ESCO2 are homologs of the S. cerevisiae acetyltransferase Eco1, essential for viability in yeast. ESCO1 and ESCO2 share sequence homology in the C-terminal region, consisting of a H2C2 zinc finger motif and an acetyltransferase domain (Hou and Zou 2005). Both ESCO1 and ESCO2 acetylate the cohesin subunit SMC3 on two lysine residues, K105 and K106 (Zhang et al. 2008), an important step in the establishment of sister-chromatid cohesion during the S-phase of the cell cycle. These dual acetylations on SMC3 are deacetylated by HDAC8 after the cohesin removal from chromatin for the dissociation and recycling of cohesin subunits (Deardorff et al. 2012). ESCO1 and ESCO2 differ in their N-termini, which are necessary for chromatin binding, and may perform distinct functions in sister chromatid cohesion (Hou and Zou 2005), as suggested by the study of Esco2 knockout mice (Whelan et al. 2012).
Reaction - small molecule participants:
CoA-SH [nucleoplasm]
Ac-CoA [nucleoplasm]
Reactome.org reaction link: R-HSA-2468039
======
Reaction input - small molecules:
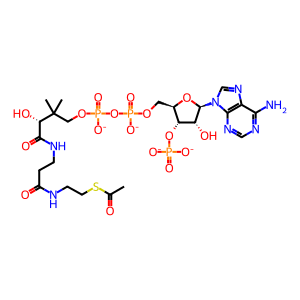
acetyl-CoA(4-)
Reaction output - small molecules:
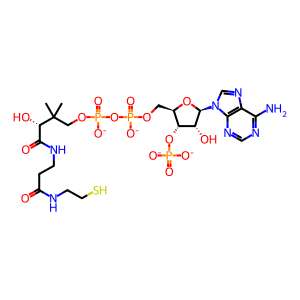
coenzyme A(4-)
Reactome.org link: R-HSA-2468039