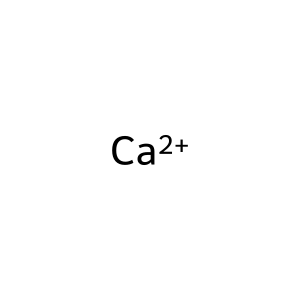Reaction: RYR tetramers transport Ca2+ from sarcoplasmic reticulum lumen to cytosol
- in pathway: Stimuli-sensing channels
Ryanodine receptors (RYRs) are located in the sarcoplasmic reticulum (SR) membrane and mediate the release of Ca2+ from intracellular stores during excitation-contraction (EC) coupling in both cardiac and skeletal muscle. RYRs are the largest known ion channels (>2MDa) and are functional in their homotetrameric forms. There are three mammalian isoforms (RYR1-3); RYR1 is prominent in skeletal muscle (Zorzato et al. 1990), RYR2 in cardiac muscle (Tunwell et al. 1996) and RYR3 is found in the brain (Nakashima et al. 1997, Lanner et al. 2010). The function of RYRs are controlled by peptidyl-prolyl cis-trans isomerase (FKBP1B), intracellular Ca2+-binding proteins calsequestrin 1 and 2 (CASQ1 and 2) and the anchoring proteins triadin (TRDN) and junctin. Together, they make up the Ca2+-release complex. CASQ1 and 2 buffer intra-SR Ca2+ stores in skeletal muscle and cardiac muscle respectively (Fujii et al. 1990, Kim et al. 2007). When Ca2+ concentrations reach 1mM, CASQs polymerise (Kim et al. 2007) and can attach to one end of RYRs, mediated by anchoring proteins TRDN and junctin (Taske et al. 1995). By sequestering Ca2+ ions, CASQs can inhibit RYRs function (Beard et al. 2004, Beard et al. 2009a, Beard et al. 2009b).
A member of the intracellular Cl- channel protein family, CLIC2, has also been determined to inhibit RYR-mediated Ca2+ transport (Board et al. 2004), potentially playing a role in the homeostasis of Ca2+ release from intracellular stores. Inhibition is thought to be via reducing activation of the channels by their primary endogenous cytoplasmic ligands, ATP and Ca2+ (Dulhunty et al. 2005). Protein kinase A (PKA) phosphorylation of RYR2 dissociates FKBP1B and results in defective channel function (Marx et al. 2000). The penta-EF hand protein sorcin (SRI) can modulate Ca2+-induced calcium-release in the heart via the interaction with several Ca2+ channels such as RYRs. A natural ligand, F112L, impairs this modulating activity (Franceschini et al. 2008). Calmodulin (CALM1) is considered a gatekeeper of RYR2. CALM1 acts directly by binding to RYR2 at residues 3583–3603, inhibiting RYR2 both at physiological and higher, pathological Ca2+ concentrations (Smith et al. 1989, Ono et al. 2010).
A member of the intracellular Cl- channel protein family, CLIC2, has also been determined to inhibit RYR-mediated Ca2+ transport (Board et al. 2004), potentially playing a role in the homeostasis of Ca2+ release from intracellular stores. Inhibition is thought to be via reducing activation of the channels by their primary endogenous cytoplasmic ligands, ATP and Ca2+ (Dulhunty et al. 2005). Protein kinase A (PKA) phosphorylation of RYR2 dissociates FKBP1B and results in defective channel function (Marx et al. 2000). The penta-EF hand protein sorcin (SRI) can modulate Ca2+-induced calcium-release in the heart via the interaction with several Ca2+ channels such as RYRs. A natural ligand, F112L, impairs this modulating activity (Franceschini et al. 2008). Calmodulin (CALM1) is considered a gatekeeper of RYR2. CALM1 acts directly by binding to RYR2 at residues 3583–3603, inhibiting RYR2 both at physiological and higher, pathological Ca2+ concentrations (Smith et al. 1989, Ono et al. 2010).
Reaction - small molecule participants:
Ca2+ [cytosol]
Ca2+ [sarcoplasmic reticulum lumen]
Ca2+ [cytosol]
Ca2+ [sarcoplasmic reticulum lumen]
Reactome.org reaction link: R-HSA-2855020
======
Reaction input - small molecules:
calcium(2+)
calcium(2+)
Reaction output - small molecules:
calcium(2+)
calcium(2+)
Reactome.org link: R-HSA-2855020