Reaction: CDK1 phosphorylates NUP98
- in pathway: Nuclear Pore Complex (NPC) Disassembly
CDK1 activity promotes the nuclear pore complex (NPC) disassembly in mitosis (Muhlhausser and Kutay 2007). While NUP98 is probably not the only nucleoporin phosphorylated by CDK1 at mitotic entry, NUP98 is the best characterized CDK1 target among nuclear pore complex components. NUP98 threonine residues T529, T536, and T653, as well as serine residues S595 and S606 were found to be phosphorylated when NUP98 was isolated from mitotic HeLa cells (human cervical carcinoma cell line); these five sites match the CDK1 target site consensus and are phosphorylated by CDK1:CCNB in vitro (Laurell et al. 2011). The NUP98 splicing isoform NUP98-4 was used in the study by Laurell et al. 2011 and the indicated positions of phosphorylated amino acid residues refer to this isoform. An additional splicing isoform NUP98-3, the product of an alternative splicing site in exon10 of the NUP98 gene, which is 17 amino acids longer than NUP98-4, could also be a part of the NPC. CDK1-phosphorylated residues in NUP98-3 would be threonines T546, T553 and T670, and serines S612 and S623.
Reaction - small molecule participants:
ADP [cytosol]
ATP [cytosol]
Reactome.org reaction link: R-HSA-2990882
======
Reaction input - small molecules:
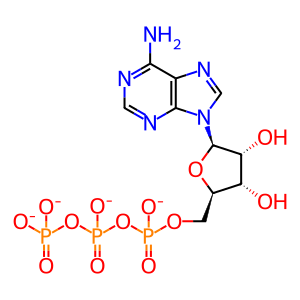
ATP(4-)
Reaction output - small molecules:
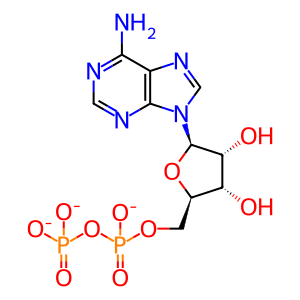
ADP(3-)
Reactome.org link: R-HSA-2990882