Reaction: Tankyrase ADP-ribosylates AXIN
- in pathway: Degradation of AXIN
TNKS1 and 2 function redundantly to control AXIN protein levels through the addition of poly-ADP-ribosyl groups (PARSylation), which may lead to subsequent ubiquitination and degradation by the proteasome. In HEK293, SW480 and breast cancer cell lines, depletion of TNKS1 and 2 increases the protein levels of AXIN1 and AXIN2 resulting in increased beta-catenin phosphorylation, decreased beta-catenin abundance and decreased expression of WNT targets and WNT-responsive reporters (Huang et al, 2009; Callow et al, 2011; Waaler et al, 2012; Bao et al, 2012). In vitro, TNKS2 catalyzes the addition of ADP-ribosyl groups to the TBD fragment of AXIN1, while in vivo, both exogenous GST-AXIN1 and endogenous AXIN1 are PARSylated in a TNKS-dependent manner (Huang et al, 2009; Callow et al, 2011; Zhang et al, 2011). PARSylation is likely required for the subsequent proteasome-mediated degradation of AXIN, as the increase in levels of polyubiquitinated AXIN1 and 2 seen upon treatment of cells with the proteasome inhibitor MG132 is lost if cells are simultaneously treated with an inhibitor of TNKS1 and 2 (Huang et al, 2009). Although in this reaction, TNKS is shown PARSylating unbound AXIN, it is likely that this regulation occurs at the level of the destruction complex. Also not shown in this reaction is the ability of TNKS to catalyze autoPARSylation reactions, which ultimately lead to its own degradation (Yeh et al, 2006; Huang et al, 2009; Zhang et al, 2011).
Reaction - small molecule participants:
NAM [cytosol]
NAD+ [cytosol]
Reactome.org reaction link: R-HSA-3640858
======
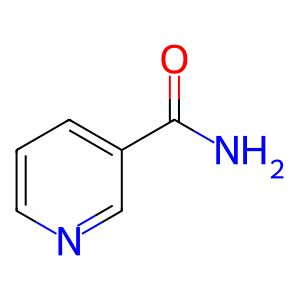
Reaction input - small molecules:
NAD(1-)
Reaction output - small molecules:
nicotinamide
Reactome.org link: R-HSA-3640858