Reaction: CREBBP acetylates histone H2B, H3, H4
- in pathway: HATs acetylate histones
CREBBP (CBP) is named after its interaction with the CRE-binding protein CREB, though it interacts with many other proteins. It is thought to act as an integrator of signals from various pathways (Goodman & Smolik 2000), which compete for a limited amount of nuclear CREBBP. CREBBP and EP300 (p300) are closely related and have overlapping functions but also unique properties, particularly in vivo (Kalkhoven 2004). Both proteins form a physical bridge between DNA-binding transcription factors and the RNA polymerase II complex. Histones are believed to be the main acetylation targets of CREBBP and EP300, but their ability to acetylate and thereby regulate transcription factors such as p53 (Gu & Roeder 1997) is considered significant additional function (Kasper et al. 2006).
CREBBP has intrinsic histone acetyltransferase (HAT) activity on lysine-13 of H2B, lysine-15 of H3 and lysine-9 of H4 (Bannister & Kouzarides 1996, Rekowski & Giannis 2010, Barrett et al. 2011).
Homozygous knockout of CREBBP results in embryonic lethality (Tanaka et al. 1997). Focal deletion of CREBBP demonstrates that it is critical for the in vivo acetylation of lysines on histones H2B, H3 and H4, and cannot be compensated for by the p300 (Barrett et al. 2011).
Genomic aberrations in CREBBP are associated with Rubinstein-Taybi syndrome (Torress et al. 2013).
N.B. Coordinates of post-translational modifications described here follow UniProt standard practice whereby coordinates refer to the translated protein before any further processing. Histone literature typically refers to coordinates of the protein after the initiating methionine has been removed. Therefore the coordinates of post-translated residues in the Reactome database and described here are frequently +1 when compared with the literature.
CREBBP has intrinsic histone acetyltransferase (HAT) activity on lysine-13 of H2B, lysine-15 of H3 and lysine-9 of H4 (Bannister & Kouzarides 1996, Rekowski & Giannis 2010, Barrett et al. 2011).
Homozygous knockout of CREBBP results in embryonic lethality (Tanaka et al. 1997). Focal deletion of CREBBP demonstrates that it is critical for the in vivo acetylation of lysines on histones H2B, H3 and H4, and cannot be compensated for by the p300 (Barrett et al. 2011).
Genomic aberrations in CREBBP are associated with Rubinstein-Taybi syndrome (Torress et al. 2013).
N.B. Coordinates of post-translational modifications described here follow UniProt standard practice whereby coordinates refer to the translated protein before any further processing. Histone literature typically refers to coordinates of the protein after the initiating methionine has been removed. Therefore the coordinates of post-translated residues in the Reactome database and described here are frequently +1 when compared with the literature.
Reaction - small molecule participants:
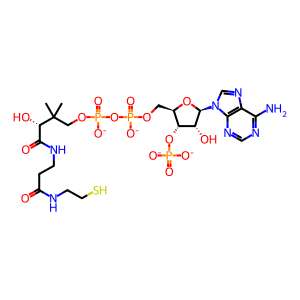
CoA-SH [nucleoplasm]
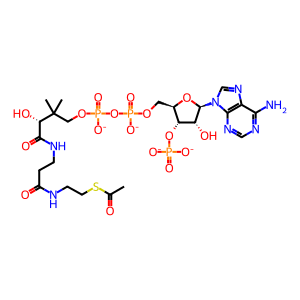
Ac-CoA [nucleoplasm]
Reactome.org reaction link: R-HSA-3697008
======
Reaction input - small molecules:
acetyl-CoA(4-)
Reaction output - small molecules:
coenzyme A(4-)
Reactome.org link: R-HSA-3697008