Reaction: HDAC1:2-containing complex deacetylate histones
- in pathway: HDACs deacetylate histones
HDAC1 and HDAC2 interact to form the catalytic core of several multisubunit complexes including the Sin3, nucleosome remodeling deacetylase (NuRD) and corepressor of REST (CoREST) complexes (Grozinger & Schreiber 2002). A 'core complex' of HDAC1/2 and the histone binding proteins RBBP7 (RbAp46) and RBBP4 (RbAp48), has been described in vivo and in vitro (Zhang et al. 1999). The Sin3 complex consists of this core complex plus SAP18 and SAP30, which appear to aid in stabilizing the protein associations and Sin3A, which serves as a scaffold for assembly of the complex and its interaction with various DNA binding proteins (Ayer 1999). Mammals express two Sin3 proteins, Sin3A and Sin3B. The recognized Sin3A core complex contains the HDAC1-2 catalytic core, SAP18 (Zhang et al. 1997), SAP30 (Zhang et al. 1998), RBBP7/4 (Ahringer 2000), SUDS3 (SAP45, SDS3) (Alland et al. 2002), ARID4B (SAP180) and SAP130 (Fleischer et al. 2003). Additional members are BRMS1 (breast cancer metastasis suppressor 1), ARID4A (Rb-binding protein 1) (Meehan et al. 2004) and SAP30L (Viiri et al. 2006). The Sin3A complex preferentially binds to hypoacetylated histones through the RBBP7/4 subunits (Vermeulen et al. 2004, Yoon et al. 2005). It can also be recruited to chromatin through the H3K4-di/trimethyl mark by ING1/2 (Shi et al. 2006, Pena et al. 2008).
The Sin3B complex shares some subunits in common with the Sin3A complex but may also contain distinct subunits (Le Guezennec et al. 2006a).
The NuRD complex contains a core histone deacetylase complex that consists of the HDAC1-2 catalytic core plus RBBP7 (RbAp46) and RBBP4 (RbAp48) (Ahringer 2000). The largest and key component is the Mi-2 remodelling subunit (dermatomyositis-specific autoantigen), which contains the ATPase/chromatin remodelling activity and physically associates with the other components. Mammals have two Mi-2 proteins: CHD3 (Mi-2alpha), and CHD4 (Mi-2 beta) (Seelig et al. 1996). CHD4 (Mi-2 beta) is the form predominantly associated with the NuRD complex (Zhang et al. 1998, Feng & Zhang 2001), although CHD3 is a member of the NuRD complex in a variety of human cell lines (Le Guezennec et al. 2006b). It is not clear whether functional differences exist between CHD3 and CHD4-containing complexes (McDonel et al. 2009). Further components are MBD3 (methyl CpG-binding domain 3), and a metastasis-associated (MTA) protein subunit. MTA subunits (e.g. Mta1, Mta2 or Mta3) appear to be mutually exclusive, possibly contributing to functional diversity of NuRD complexes (Bowen et al. 2004, Fujita et al. 2004). GATAD2A and GATAD2B proteins (formerly known as p66alpha and p66beta) are often reported as members of NuRD. MBD3 can be replaced by related protein MBD2, forming the MeCP1 complex (Feng & Zhang 2001, Le Guezennec et al. 2006b). The MeCP1 complex represents only a small proportion of the total NuRD complex in mammalian cells (Refs. in McDonel et al. 2009); MBD2 has been shown to be dispensable for normal mammalian development (Hendrich et al. 2001).
NuRD is recruited via MDB3 for DNA methylation-dependent gene silencing. It associates with MeCP1 (methyl CpG-binding protein 1) and MeCP2 to provide an intimate connection with DNA methylation (Denslow & Wade 2007, Klose & Bird 2006).
The CoREST complex minimally contains the HDAC1-2 catalytic core, REST (RE1-silencing transcription factor), RCOR1 (CoREST, KIAA0071) and KDM1A (BHC110, LSD1) (Andres et al. 1999, Humphrey et al. 2001). The BRAF–HDAC (BHC) complex consists of HDAC1-2, RCOR1, KDM1A, HMG20B (BRAF35) and PHF21A (BHC80) (Hakimi et al. 2002, Yang & Seto 2008).
This reaction represents a theoretical complete deacetylation of histone.
The Sin3B complex shares some subunits in common with the Sin3A complex but may also contain distinct subunits (Le Guezennec et al. 2006a).
The NuRD complex contains a core histone deacetylase complex that consists of the HDAC1-2 catalytic core plus RBBP7 (RbAp46) and RBBP4 (RbAp48) (Ahringer 2000). The largest and key component is the Mi-2 remodelling subunit (dermatomyositis-specific autoantigen), which contains the ATPase/chromatin remodelling activity and physically associates with the other components. Mammals have two Mi-2 proteins: CHD3 (Mi-2alpha), and CHD4 (Mi-2 beta) (Seelig et al. 1996). CHD4 (Mi-2 beta) is the form predominantly associated with the NuRD complex (Zhang et al. 1998, Feng & Zhang 2001), although CHD3 is a member of the NuRD complex in a variety of human cell lines (Le Guezennec et al. 2006b). It is not clear whether functional differences exist between CHD3 and CHD4-containing complexes (McDonel et al. 2009). Further components are MBD3 (methyl CpG-binding domain 3), and a metastasis-associated (MTA) protein subunit. MTA subunits (e.g. Mta1, Mta2 or Mta3) appear to be mutually exclusive, possibly contributing to functional diversity of NuRD complexes (Bowen et al. 2004, Fujita et al. 2004). GATAD2A and GATAD2B proteins (formerly known as p66alpha and p66beta) are often reported as members of NuRD. MBD3 can be replaced by related protein MBD2, forming the MeCP1 complex (Feng & Zhang 2001, Le Guezennec et al. 2006b). The MeCP1 complex represents only a small proportion of the total NuRD complex in mammalian cells (Refs. in McDonel et al. 2009); MBD2 has been shown to be dispensable for normal mammalian development (Hendrich et al. 2001).
NuRD is recruited via MDB3 for DNA methylation-dependent gene silencing. It associates with MeCP1 (methyl CpG-binding protein 1) and MeCP2 to provide an intimate connection with DNA methylation (Denslow & Wade 2007, Klose & Bird 2006).
The CoREST complex minimally contains the HDAC1-2 catalytic core, REST (RE1-silencing transcription factor), RCOR1 (CoREST, KIAA0071) and KDM1A (BHC110, LSD1) (Andres et al. 1999, Humphrey et al. 2001). The BRAF–HDAC (BHC) complex consists of HDAC1-2, RCOR1, KDM1A, HMG20B (BRAF35) and PHF21A (BHC80) (Hakimi et al. 2002, Yang & Seto 2008).
This reaction represents a theoretical complete deacetylation of histone.
Reaction - small molecule participants:
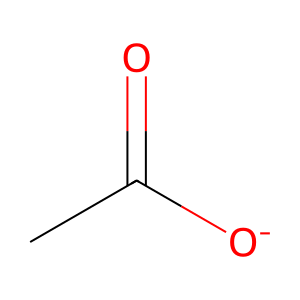
acetate [nucleoplasm]
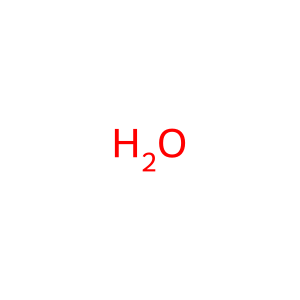
H2O [nucleoplasm]
Reactome.org reaction link: R-HSA-3769447
======
Reaction input - small molecules:
water
Reaction output - small molecules:
acetate
Reactome.org link: R-HSA-3769447