Reaction: G alpha (i) auto-inactivates by hydrolysing GTP to GDP
- in pathway: G alpha (i) signalling events
When a ligand activates a G protein-coupled receptor, it induces a conformational change in the receptor (a change in shape) that allows the receptor to function as a guanine nucleotide exchange factor (GEF), stimulating the exchange of GDP for GTP on the G alpha subunit. In the traditional view of heterotrimeric protein activation, this exchange triggers the dissociation of the now active G alpha subunit from the beta:gamma dimer, initiating downstream signalling events. The G alpha subunit has intrinsic GTPase activity and will eventually hydrolyze the attached GTP to GDP, allowing reassociation with G beta:gamma. Additional GTPase-activating proteins (GAPs) stimulate the GTPase activity of G alpha, leading to more rapid termination of the transduced signal. In some cases the downstream effector may have GAP activity, helping to deactivate the pathway. This is the case for phospholipase C beta, which possesses GAP activity within its C-terminal region (Kleuss et al. 1994).
Reaction - small molecule participants:
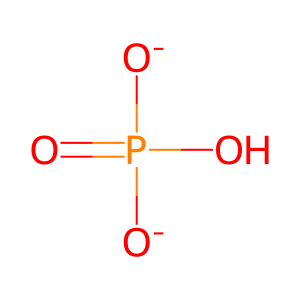
Pi [cytosol]
Reactome.org reaction link: R-HSA-392212
======
Reaction input - small molecules:
Reaction output - small molecules:
hydrogenphosphate
Reactome.org link: R-HSA-392212