Reaction: EPH receptors autophosphorylate
- in pathway: EPH-Ephrin signaling
Following ligand binding, EPH signaling is initiated through autophosphorylation. The cytoplasmic domain of EPH receptors can be divided into four functional units; the juxtamembrane region, a tyrosine kinase domain, a sterile alpha-motif (SAM) and a PDZ-domain binding motif. Multiple in vivo tyrosine phosphorylation sites were identified in the juxtamembrane region, kinase domain, and carboxy-terminal tail of EPH receptors. EPH receptors transduce forward signals into the cell through phosphorylation of these tyrosine (Y) residues. Two autophosphorylation sites within the juxtamembrane region (example phosphorylation sites being Y596 and 602 on EPHA4 and Y596 and 602 on EPHB2), and a Y residue within the kinase domain activation segment are identified as the key phosphorylation sites required for the catalytic activity of these EPH receptors. These Y residues are remarkably conserved between the EPHA and EPHB receptors. Substitution of these conserved Y residues in full-length EPHB2 leads to a reduction in ligand-induced kinase activity and EFN-stimulated tyrosine phosphorylation, suggesting that juxtamembrane Y residues may serve a regulatory function in addition to acting as docking sites for downstream targets. These autophosphorylated residues have been shown to interact with a number of proteins including Ras GTPase-activating protein (RasGAP), the p85 subunit of phosphatidylinositol 3-kinase, Src family kinases, the adapter protein NCK, and SHEP-1 (Binns et al. 2000).
Reaction - small molecule participants:
ADP [cytosol]
ATP [cytosol]
Reactome.org reaction link: R-HSA-3928578
======
Reaction input - small molecules:
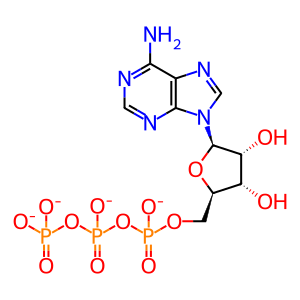
ATP(4-)
Reaction output - small molecules:
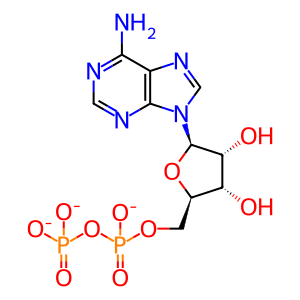
ADP(3-)
Reactome.org link: R-HSA-3928578