Reaction: PRKG is activated by binding to high levels of cGMP in the absence of WNT signaling
- in pathway: Ca2+ pathway
Active PKG homodimers restrict Ca2+ release in the absence of stimulation by phosphorylating a component of the IP3 receptor complex. PKG homodimers are activated at high (sub- to micromolar) concentrations of cGMP. Upon binding of cGMP to the high and low affinity sites in the regulatory domain, an allosteric change in secondary structure makes the catalytic site accessible to substrate (reviewed in Hoffman, 2005). WNT5A signaling through FZD2 in mouse F9 teratocarcinoma cells results in a PDE6-dependent decrease in cGMP levels. Under low cGMP conditions, the N-terminal domain of PKG occludes the catalytic site, reducing PKG activity and promoting Ca2+ release through the IP3 receptor (Ahumada et al, 2002; Ma and Yang, 2006).
Reaction - small molecule participants:
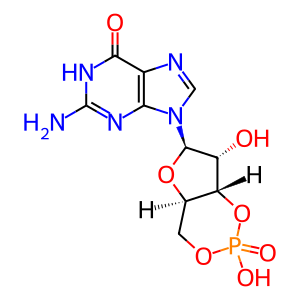
cGMP [cytosol]
Reactome.org reaction link: R-HSA-4551453
======
Reaction input - small molecules:
3',5'-cyclic GMP
Reaction output - small molecules:
Reactome.org link: R-HSA-4551453