Reaction: Defective ALG6 does not add glucose to the N-glycan precursor
- in pathway: Defective ALG6 causes CDG-1c
Dolichyl pyrophosphate Man9GlcNAc2 alpha-1,3-glucosyltransferase (ALG6) normally adds the first glucose moiety to the lipid-linked oligosaccharide precursor (LLO aka N-glycan precursor) which is required for subsequent N-glycosylation of proteins (Imbach et al. 1999). Defects in ALG6 can cause congenital disorder of glycosylation 1c (ALG6-CDG, CDG-1c; MIM:603147), a multisystem disorder characterised by under-glycosylated serum glycoproteins (Imbach et al. 1999, Imbach et al. 2000, Westphal et al. 2000, Sun et al. 2005). ALG6 deficiency is accompanied by an accumulation of the N-glycan precursor (GlcNAc)2 (Man)9 (PP-Dol)1 (Imbach et al. 1999). CDG type 1 diseases result in a wide variety of clinical features, such as defects in the nervous system development, psychomotor retardation, dysmorphic features, hypotonia, coagulation disorders, and immunodeficiency. Mutations that can cause ALG6-CDG are A333V and S478P. The A333V mutation is the most commom mutation seen in ALG6-CDG patients. These mutations result in altered activity of ALG6 but don't completely abolish its activity (Imbach et al. 1999, Imbach et al. 2000, Dercksen et al. 2013). A c.257+5G>A splice site mutation (not shown here) that causes exon 3 skipping leads to a nonfunctional protein (Imbach et al. 2000, Westphal et al. 2000). Two more mutations can cause the build up of the N-glycan precursor (GlcNAc)2 (Man)9 (PP-Dol)1; a three bp deletion (897-899delAAT) in exon 9 and an intronic
mutation (680+2T>G) in intron 7 (neither shown here). Transduction of patient fibroblasts with a lentivirus carrying wildtype hALG6 improved the biochemical phenotype of the cells, confirming that these two mutations are disease-causing (Sun et al. 2005).
mutation (680+2T>G) in intron 7 (neither shown here). Transduction of patient fibroblasts with a lentivirus carrying wildtype hALG6 improved the biochemical phenotype of the cells, confirming that these two mutations are disease-causing (Sun et al. 2005).
Reaction - small molecule participants:
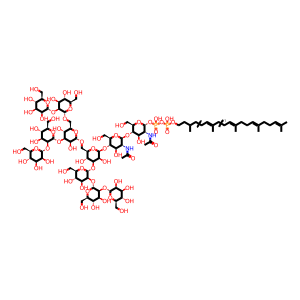
(GlcNAc)2 (Man)9 (PP-Dol)1 [integral component of lumenal side of endoplasmic reticulum membrane]
DbGP [integral component of lumenal side of endoplasmic reticulum membrane]
Reactome.org reaction link: R-HSA-4724291
======
Reaction input - small molecules:
alpha-Man-(1->2)-alpha-Man-(1->2)-alpha-Man-(1->3)-[alpha-Man-(1->2)-alpha-Man-(1->3)-[alpha-Man-(1->2)-alpha-Man-(1->6)]-alpha-Man-(1->6)]-beta-Man-(1->4)-beta-GlcNAc-(1->4)-alpha-GlcNAc(PP-Dol)
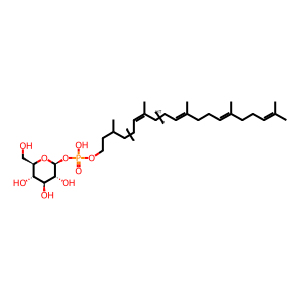
dolichyl beta-D-glucosyl phosphate
Reaction output - small molecules:
Reactome.org link: R-HSA-4724291