Reaction: Phosphorylation of HSF1 at Ser230 induces transactivation
- in pathway: HSF1-dependent transactivation
The transcriptional activity of HSF1 has been shown to be controlled by the regulatory domain composed of amino acids 221-310 (Green M et al. 1995; Zuo J et al. 1995; Newton EM et al., 1996). Ser230 is located in this regulatory domain of HSF1 and is constitutively and stress-inducibly phosphorylated (Holmberg CI et al. 2001). Analyses with phosphopeptide-specific antibody and site-directed mutagenesis revealed that phosphorylation at Ser230 enhanced the inducible HSF1 transcriptional activity in heat-shocked human K562 erythroleukemia and HeLa cells (Holmberg CI et al 2001). Active calcium/calmodulin-dependent protein kinase II (CaMKII) was shown to phosphorylate HSF1 at Ser230 in vitro. Moreover, CaMKII enhanced heat-induced tranactivating capacity of HSF1 and the level of endogenous Ser230 phosphorylation in K562 cells transfected with active CaMKII together with a CAT reporter plasmid containing the proximal HSE of human hsp70 promoter. Thus, CaMKII signaling may be involved in the positive regulation of HSF1-mediated transactivation. However, the possibility that other protein kinases might also phosphorylate Ser230 in vivo should not be excluded (Holmberg CI et al 2001).
Reaction - small molecule participants:
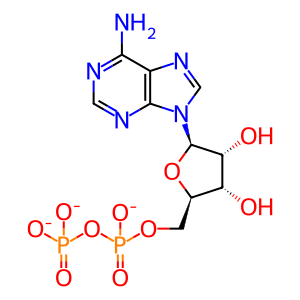
ADP [nucleoplasm]
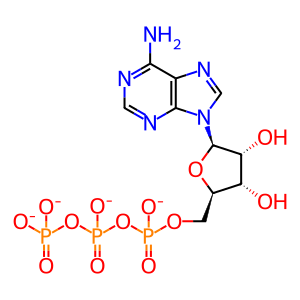
ATP [nucleoplasm]
Reactome.org reaction link: R-HSA-5082387
======
Reaction input - small molecules:
ATP(4-)
Reaction output - small molecules:
ADP(3-)
Reactome.org link: R-HSA-5082387