Reaction: BCO2:Fe2+ cleaves betaC to APO10al and bION
- in pathway: Retinoid metabolism and transport
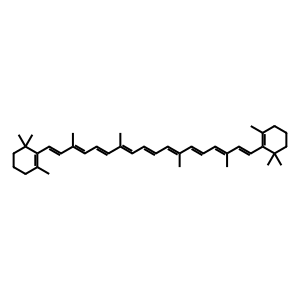
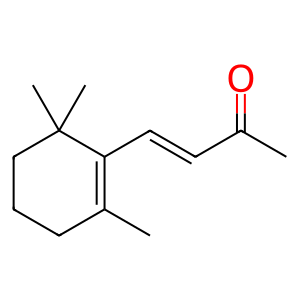
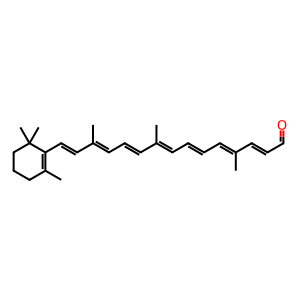
Beta,beta-carotene 9',10'-oxygenase (BCO2) is able to eccentrically cleave carotenoids to produce long chain (>C20) apocarotenoids (Amengual et al. 2011). This is in contrast to the other provitamin A-converting enzyme, BCMO1 which is able to symmetrically cleave carotenoids to produce apocarotenoids of C20 length, such as all-trans-retinal (atRAL). BCMO1 is the main enzyme involved in retinoid homeostasis and resides in the cytosol whereas BCO2 resides in the mitchondrion, has broad substrate activity and is proposed to provide an alternative, minor route for retinoid production. How apocarotenoids produced by BCO2 cleavage are utilised is the subject of further investigation (Amengual et al. 2013). Being in the mitochondrion, BCO2 is able to degrade carotenoids which, if otherwise allowed to accumulate, are implicated in oxidative damage to the cell (Amengual et al. 2011). In this example, beta-carotene (betaC) is cleaved by BCO2 to produce beta-apo-10'-carotenal (APO10al) and beta-ionone (bION) in an enterocyte cell. Carotenoids, such as betaC, can also be metabolised in many other cell types including hepatocytes and stellate cells of the liver.
Reaction - small molecule participants:
bION [mitochondrial matrix]
APO10al [mitochondrial matrix]
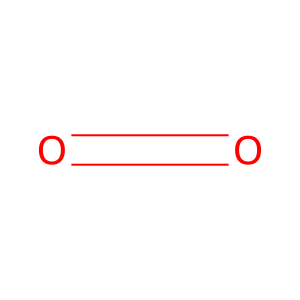
O2 [mitochondrial matrix]
betaC [mitochondrial matrix]
Reactome.org reaction link: R-HSA-5164399
======
Reaction input - small molecules:
dioxygen
beta-carotene
Reaction output - small molecules:
beta-ionone
10'-apo-beta-carotenal
Reactome.org link: R-HSA-5164399