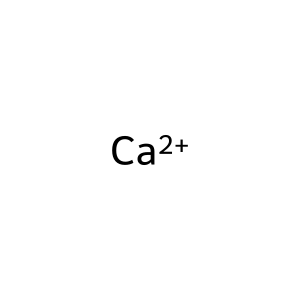Reaction: DAG and Ca+2 bind to PKC and tether it to membrane
- in pathway: Depolymerization of the Nuclear Lamina
PKC contains an N-terminal C2 like domain, a pseudosubstrate (PS), DAG binding (C1) domain and a C-terminal kinase domain. The PS sequence resembles an ideal substrate with the exception that it contains an alanine residue instead of a substrate serine residue, is bound to the kinase domain in the resting state. As a result, PKC is maintained in a closed inactive state, which is inaccessible to cellular substrates (Colon-Gonzalez & Kazanietz 2006). Diacylglycerol (DAG) produced by activated lipins (LPIN1, LPIN2, LPIN3) leads to the activation of PKC (PRKCA and PRKCB) and their translocation from the nucleoplasm to the nuclear envelope where they can phosphorylate lamins (Mall et al. 2012). PKCs are tethered to the membrane through DAG binding to the C1 domain and this confers a high-affinity interaction between PKC and the membrane. This leads to a massive conformational change that releases the PS domain from the catalytic site and the system becomes both competent and accessible (Colon-Gonzalez & Kazanietz 2006).
Reaction - small molecule participants:
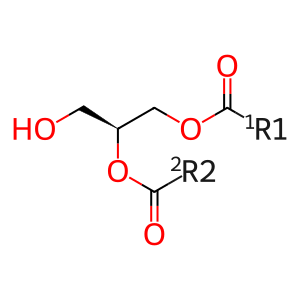
DAG [nuclear envelope]
Ca2+ [nucleoplasm]
Reactome.org reaction link: R-HSA-5223304
======
Reaction input - small molecules:
1,2-diacyl-sn-glycerol
calcium(2+)
Reaction output - small molecules:
Reactome.org link: R-HSA-5223304