Reaction: AXIN is phosphorylated in the destruction complex
- in pathway: Degradation of beta-catenin by the destruction complex
In the absence of WNT signal, AXIN is a phosphoprotein; candidate kinases include both GSK3beta and CK1 (Ikeda et al, 1998; Willert et al, 1999; Jho et al, 1999; Yamamoto et al, 1999; Luo et al, 2007). Phosphorylation of AXIN is thought to increase its binding affinity for beta-catenin and GSK3beta, stabilizing the destruction complex and promoting efficient degradation of beta-catenin (Willert et al, 1999; Jho et al, 1999; Luo et al, 2007). A more recent model suggests that AXIN phosphorylation may disrupt an intramolecular interaction between its DIX domain and the beta-catenin binding region, which would otherwise keep AXIN in a 'closed' inactive state (Kim et al, 2013). Activation of the WNT pathway upon ligand binding favours dephosphorylation of AXIN by inactivating the kinases and allowing the steady state dephosphorylation by candidate phosphatases PP2A and PP1 to predominate (Willert et al, 1999; Luo et al, 2007; reviewed in Saito-Diaz et al, 2013).
Reaction - small molecule participants:
ADP [cytosol]
ATP [cytosol]
Reactome.org reaction link: R-HSA-5229343
======
Reaction input - small molecules:
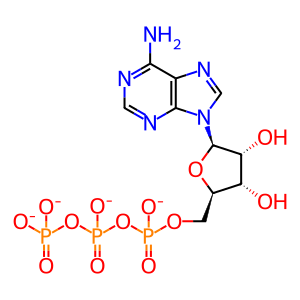
ATP(4-)
Reaction output - small molecules:
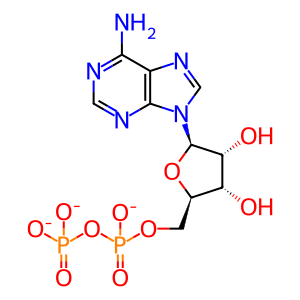
ADP(3-)
Reactome.org link: R-HSA-5229343