Reaction: HSP40s activate intrinsic ATPase activity of HSP70s in the cytosol
- in pathway: Regulation of HSF1-mediated heat shock response
Heat-shock 70kDa proteins (HSP70s) are a family of conserved, ubiquitously expressed heat-shock proteins which play important roles in protein folding and in protecting cells from stress. They possess three functional domains; an N-terminal ATPase domain, a substrate binding domain and a C-terminal domain. HSP70s are bound to either ATP or ADP. In the ATP-bound state, HSP70s do not interact with a substrate peptide as the C-terminal domain (which acts as a lid) is "open", allowing peptides to bind to the substrate binding domain but then be released very rapidily. However, a substrate peptide in the binding domain can stimulate the intrinsic ATPase activity of HSP70s, hydrolysing ATP to ADP. With ADP bound, the C-terminal domain of HSP70s closes around the peptide, effectively trapping the peptide.
Intrinsic ATPase activity proceeds relatively slowly but can be dramatically increased by binding of J-domain chaperones such as HSP40s. These are eukaryotic orthologues of the DnaJ cochaperones found in prokaryotes. The human HSP40s that are able to modulate intrinsic ATPase activity of HSP70s are DNAJB1, B6, C2 and C7 (Raabe & Manley 1991, Melville et al. 1999, Hanai & Mashima 2003, Izawa et al. 2000, Hundley et al. 2005, Brychzy et al. 2003). They are also able to co-localise to the nucleus with HSP70s upon heat-shock (Hattori et al. 1992).
Intrinsic ATPase activity proceeds relatively slowly but can be dramatically increased by binding of J-domain chaperones such as HSP40s. These are eukaryotic orthologues of the DnaJ cochaperones found in prokaryotes. The human HSP40s that are able to modulate intrinsic ATPase activity of HSP70s are DNAJB1, B6, C2 and C7 (Raabe & Manley 1991, Melville et al. 1999, Hanai & Mashima 2003, Izawa et al. 2000, Hundley et al. 2005, Brychzy et al. 2003). They are also able to co-localise to the nucleus with HSP70s upon heat-shock (Hattori et al. 1992).
Reaction - small molecule participants:
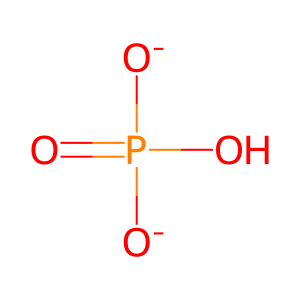
Pi [cytosol]
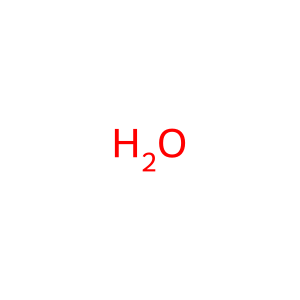
H2O [cytosol]
Reactome.org reaction link: R-HSA-5251959
======
Reaction input - small molecules:
water
Reaction output - small molecules:
hydrogenphosphate
Reactome.org link: R-HSA-5251959