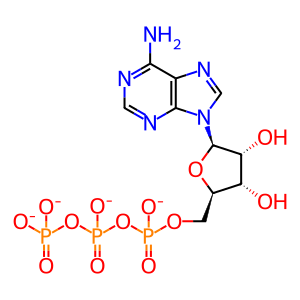
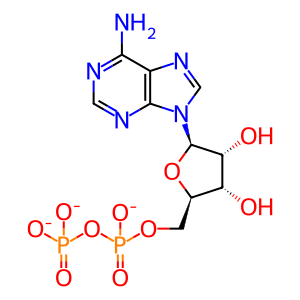
Reaction: HSP110s exchange ATP for ADP on HSP70s:ADP
- in pathway: Regulation of HSF1-mediated heat shock response
The substrate binding ability of heat-shock 70kDa proteins (HSP70s) is dependant on their bound state to either ATP or ADP. Release of a protein substrate is induced when HSP70s are bound to ATP and conversely, proteins substrates bind when HSP70s are bound to ADP. Intrinsic ATPase of HSP70s slowly hydrolyses ATP to ADP. This process can be speeded up by cochaperones such as HSP40s which stimulate ATPase activity. Nucleotide exchange factors (NEFs) regulate the lifespan of the HSP70:ADP:substrate complex by exchanging ADP for ATP, thus inducing the release of the substrate. Eukaryote NEFs include heat-shock protein 105kDa (HSPH1 aka HSP110) (Schuermann et al. 2008) and the BAG family molecular chaperone regulator (BAG) family (BAG1-5). BAGs inhibit the chaperone activity of HSP70s by promoting substrate release (Takayama et al. 1997, Takayama et al. 1999). HSC70-interacting protein (ST13 aka HIP) is a 48kDa tetrameric protein able to bind the ATPase domain of HSP70s and thought to stabilise the ADP state of HSP70s (Hohfeld et al. 1995, Prapapanich et al. 1996).
Reaction - small molecule participants:
ADP [cytosol]
ATP [cytosol]
Reactome.org reaction link: R-HSA-5252079
======
Reaction input - small molecules:
ATP(4-)
Reaction output - small molecules:
ADP(3-)
Reactome.org link: R-HSA-5252079