Reaction: Removal of the third glucose by glucosidase II and release from the chaperone
- in pathway: Calnexin/calreticulin cycle
While the protein is bound to the chaperone complex, the glycan is still accessible to glucosidase II, which eventually removes the last remaining glucose residue. This also results in breaking the interaction between the chaperone and the glycoprotein, independently of whether the latter has achieved proper folding (Pelletier MF et al, 2000). This has been interpreted as a 'timing mechanism', in which a protein has only a limited period of time to achieve correct folding when bound to the chaperone, to avoid the scenario where proteins that take too long to fold would block the availability of CNX or CRT. Proteins with folding defects get transported to the Endoplasmic Reticulum Quality Control Compartment, while proteins with correct folding are transported to the cis-Golgi where the glycan is further modified.
Reaction - small molecule participants:
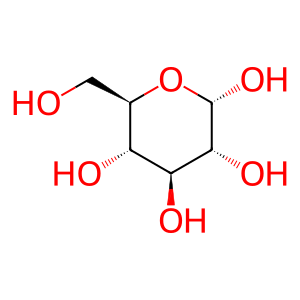
Glc [endoplasmic reticulum lumen]
Reactome.org reaction link: R-HSA-548890
======
Reaction input - small molecules:
Reaction output - small molecules:
alpha-D-glucose
Reactome.org link: R-HSA-548890