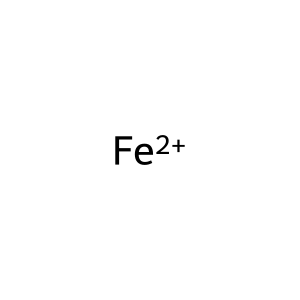Reaction: Defective CP does not oxidise Fe2+ to Fe3+
- in pathway: Defective CP causes aceruloplasminemia (ACERULOP)
Ceruloplasmin (CP), synthesised in the liver and secreted into plasma, is a copper-binding (6-7 atoms per molecule) glycoprotein involved in iron trafficking in vertebrates. CP is essential for SLC40A1 (ferroportin) stability at the cell surface, the protein that mediates iron efflux from cells. CP also possesses ferroxidase activity, which oxidises ferrous iron (Fe2+) to ferric iron (Fe3+) following its transfer out of the cell. Defects in CP (or indeed SLC40A1) can lead to the phenotype of iron overload as seen in the disorder aceruloplasminemia (ACERULOP; MIM:604290). It is a rare autosomal recessive disorder of iron metabolism characterised by iron accumulation mainly in the brain, but also in liver, pancreas and retina. Patients develop retinal degeneration, diabetes mellitus and neurological disturbance.
Mutations that can cause ACERULOP include W858*, E797Rfs*12, D411Tfs*36 and 778fs*12 (Takahashi et al. 1996, Logan et al. 1994, Okamoto et al. 1996, Harris et al. 1995).
Mutations that can cause ACERULOP include W858*, E797Rfs*12, D411Tfs*36 and 778fs*12 (Takahashi et al. 1996, Logan et al. 1994, Okamoto et al. 1996, Harris et al. 1995).
Reaction - small molecule participants:
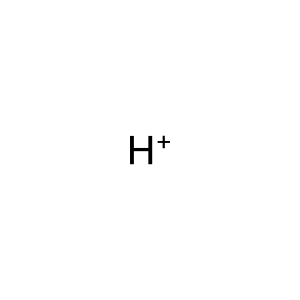
H+ [extracellular region]
Fe2+ [extracellular region]
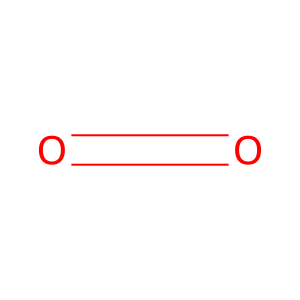
O2 [extracellular region]
Reactome.org reaction link: R-HSA-5621402
======
Reaction input - small molecules:
hydron
iron(2+)
dioxygen
Reaction output - small molecules:
Reactome.org link: R-HSA-5621402