Reaction: ULK3 phosphorylates GLI
- in pathway: Hedgehog 'on' state
Dissociation from SUFU allows the STK36/dFu homologue ULK3 to phosphorylate full-length GLI proteins. Phosphorylation promotes the nuclear translocation of the proteins and stimulates the transcription factor activity as assessed by a GLI-responsive reporter gene. In vitro, ULK3 phosphorylates GLI2 with the highest efficiency, but the kinase is also able to phosphorylate GLI1 and GLI3 (Maloverjan et al, 2010a; Maloverjan et al, 2010b). ULK3 is only one of a number of kinases that have been implicated in the regulation of GLI proteins in response to pathway stimulation, and how all the putative regulators interact to control GLI transcriptional activity remains to be elucidated (Evangelista et al, 2008; Mao et al, 2002; Varjosalo et al, 2008; reviewed in Marjosalo and Piirsoo, 2012).
Reaction - small molecule participants:
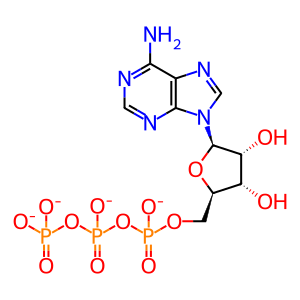
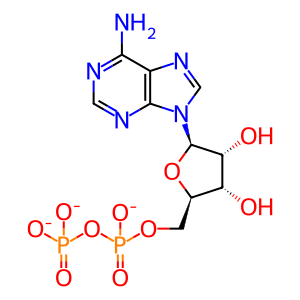
ADP [cilium]
ATP [cilium]
Reactome.org reaction link: R-HSA-5635842
======
Reaction input - small molecules:
ATP(4-)
Reaction output - small molecules:
ADP(3-)
Reactome.org link: R-HSA-5635842