Reaction: Activated FGFR2 binds PLCG1
- in pathway: Phospholipase C-mediated cascade; FGFR2
Recruitment of PLC-gamma by FGF receptors has been best studied in FGFR1c signaling, where it has been shown that autophosphorylation of Tyr766 in the C-terminal tail of FGFR1c creates a specific binding site for the SH2 domain of PLC-gamma. A mutant FGFR1c in which Y766 is replaced by phenylalanine is unable to activate PI hydrolysis and Ca2+ release in response to FGF stimulation. Membrane recruitment of PLC-gamma is also aided by binding of the Pleckstrin homology (PH) domain of this enzyme to PtIns(3,4,5) P3 molecules that are generated in response to PI-3 kinase stimulation. By sequence comparison, Y766 is conserved in all FGFR isoforms, and PLC-gamma signaling is observed, to a greater or lesser extent, downstream of all FGFR receptors upon stimulation with FGFs.
Reaction - small molecule participants:
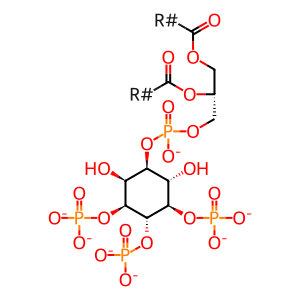
PI(3,4,5)P3 [plasma membrane]
Reactome.org reaction link: R-HSA-5654159
======
Reaction input - small molecules:
1-phosphatidyl-1D-myo-inositol 3,4,5-trisphosphate(7-)
Reaction output - small molecules:
Reactome.org link: R-HSA-5654159