Reaction: Dopaquinone, cysteine form CD isomers
- in pathway: Melanin biosynthesis
In the presence of sulfhydryl compounds such as cysteine, dopaquinone reacts to produce several cysteinyldopa (CD) isomers, 5-S-cysteinyldopa (5SCD) and 2-S-cysteinyldopa (2SCD) in 74% and 14% yields, respectively (Ito & Prota 1977, Thompson et al. 1985). 2,5-S,S'-dicysteinyldopa (DiCD) is produced in a 5% yield. Further oxidation of the thiol adducts leads to the formation of pheomelanin via benzothiazine intermediates.
Reaction - small molecule participants:
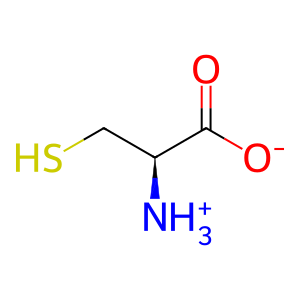
L-Cys [melanosome lumen]
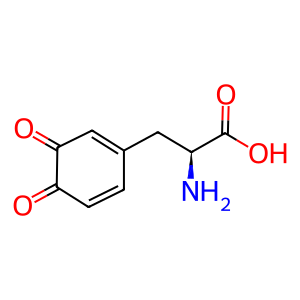
L-Dopaquinone [melanosome lumen]
Reactome.org reaction link: R-HSA-5662908
======
Reaction input - small molecules:
L-cysteine zwitterion
L-dopaquinone
Reaction output - small molecules:
Reactome.org link: R-HSA-5662908