Reaction: DHI and DHICA polymerize forming eumelanin
- in pathway: Melanin biosynthesis
Eumelanin is a stacked, aggregated oligomer, or heterogeneous polymer consisting of units representing different oxidative states of 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA), plus pyrrole units derived from their peroxidative cleavage (Meredith & Sarna 2006, Ito & Wakamatsu 2008). Eumelanin was thought to consist mostly of DHI but this was reconsidered when chemical degradation revealed that natural eumelanins include DHI and DHICA units in a nearly equal ratio (Ito 1986, d'Ischia et al. 2013). DHICA is produced by tautomerization of dopachrome.The oxidative polymerization of DHI can be catalyzed by Tyrosinase (TYR) (Tripathi et al. 1992) but may also be effectively catalysed by redox exchange with dopaquinone (Edge et al. 2006). Redox is likely to be less efficient for DHICA. In mice, the tyrosinase-related protein Tyrp1 can oxidize DHICA (Kobayashi et al. 1994) but human TYRP1 is unable to catalyze the same reaction (Boissy et al. 1998). Instead, human TYR oxidizes DHICA, DHI, tyrosine and dopa.
Reaction - small molecule participants:
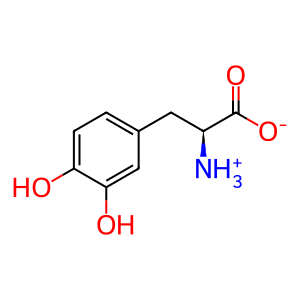
L-Dopa [melanosome lumen]
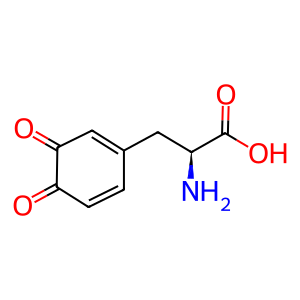
L-Dopaquinone [melanosome lumen]
Reactome.org reaction link: R-HSA-5663050
======
Reaction input - small molecules:
L-dopaquinone
Reaction output - small molecules:
L-dopa zwitterion
Reactome.org link: R-HSA-5663050