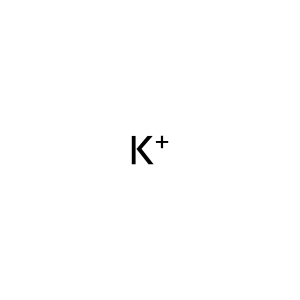Reaction: Activating ABCC8 mutants cause hyperglycemia in permanent neonatal diabetes mellitus (PNDM) and transient neonatal DM (TNDM).
- in pathway: Defective ABCC8 can cause hypo- and hyper-glycemias
Neonatal diabetes mellitus (NDM) is a rare condition defined as insulin-requiring hyperglycemia within the first month of life. About half of the neonates have a transient form that resolves at a median age of 3 months whereas the rest have a permanent form of diabetes.
ATP-binding cassette sub-family C member 8 (ABCC8) is a subunit of the beta-cell ATP-sensitive potassium channel (KATP). KATP channels play an important role in the control of insulin release. Elevation of the ATP:ADP ratio closes KATP channels leading to cellular depolarisation, calcium influx and exocytosis of insulin from its storage granules. Defects in ABCC8 can cause dysregulation of insulin secretion resulting in hyperglycemias or hypoglycemias.
Defects in ABCC8 can cause permanent neonatal diabetes mellitus (PNDM; MIM:606176). Wild-type ABCC8 confers a lower open-channel probability (Po) than activating mutations of ABCC8, where overactive KATP channels reduce insulin secretion, resulting in hyperglycemia, diagnosed within the first months of life. PNDM requires lifelong therapy. Mutations causing PNDM include P132L, L213R, N72S, A1185E and E382K (Proks et al. 2006, Babenko et al. 2006, Ellard et al. 2007).
Defects in ABCC8 can also cause transient noenatal diabetes mellitus 2 (TNDM2; MIM:610374). Babies are born with intrauterine growth retardation and present within the first 6 weeks of life with severe failure to thrive, hyperglycemia, and dehydration. The condition usually resolves within the first 6 months after birth but there is a predisposition to type 2 diabetes later in life (Naylor et al. 2011). Activating mutations causing TNDM2 are R1379C and L582V (Babenko et al. 2006). These mutations confer a higher open-channel probability (Po) than wild-type ABCC8, keeping the KATP channel open thus not allow cellular depolarisation to occur with the end result of reduced insulin secretion and hyperglycemia.
ATP-binding cassette sub-family C member 8 (ABCC8) is a subunit of the beta-cell ATP-sensitive potassium channel (KATP). KATP channels play an important role in the control of insulin release. Elevation of the ATP:ADP ratio closes KATP channels leading to cellular depolarisation, calcium influx and exocytosis of insulin from its storage granules. Defects in ABCC8 can cause dysregulation of insulin secretion resulting in hyperglycemias or hypoglycemias.
Defects in ABCC8 can cause permanent neonatal diabetes mellitus (PNDM; MIM:606176). Wild-type ABCC8 confers a lower open-channel probability (Po) than activating mutations of ABCC8, where overactive KATP channels reduce insulin secretion, resulting in hyperglycemia, diagnosed within the first months of life. PNDM requires lifelong therapy. Mutations causing PNDM include P132L, L213R, N72S, A1185E and E382K (Proks et al. 2006, Babenko et al. 2006, Ellard et al. 2007).
Defects in ABCC8 can also cause transient noenatal diabetes mellitus 2 (TNDM2; MIM:610374). Babies are born with intrauterine growth retardation and present within the first 6 weeks of life with severe failure to thrive, hyperglycemia, and dehydration. The condition usually resolves within the first 6 months after birth but there is a predisposition to type 2 diabetes later in life (Naylor et al. 2011). Activating mutations causing TNDM2 are R1379C and L582V (Babenko et al. 2006). These mutations confer a higher open-channel probability (Po) than wild-type ABCC8, keeping the KATP channel open thus not allow cellular depolarisation to occur with the end result of reduced insulin secretion and hyperglycemia.
Reaction - small molecule participants:
K+ [extracellular region]
K+ [cytosol]
Reactome.org reaction link: R-HSA-5683209
======
Reaction input - small molecules:
potassium(1+)
Reaction output - small molecules:
potassium(1+)
Reactome.org link: R-HSA-5683209