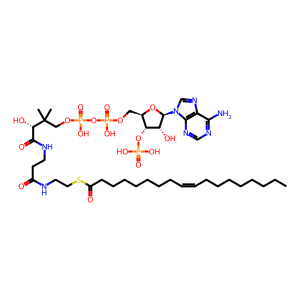
Reaction: SCD desaturates ST-CoA to OLE-CoA
- in pathway: Fatty acyl-CoA biosynthesis
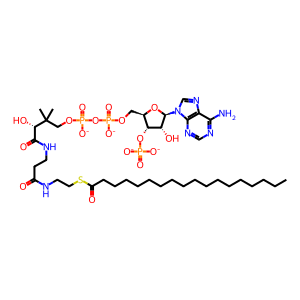
Acyl-CoA desaturase (SCD), located on the ER membrane, is the terminal enzyme of the liver microsomal stearyl-CoA desaturase system and is the rate-limiting enzyme in the cellular synthesis of monounsaturated fatty acids (MUFAs) from saturated fatty acids. SCD utilises O2 and electrons from reduced ferrocytochrome b5 (Fe(2+)Cb5) to catalyse the insertion of a double bond into a range of fatty acyl-CoA substrates. This example shows stearoyl-CoA (ST-CoA) desaturation to oleoyl-CoA (OLE-CoA) (Li et al. 1994, Zhang et al. 1999). Studies of tagged recombinant enzyme overexpressed in transiently transfected cells suggest that the enzyme forms dimers and higher oligomers (Zhang et al. 2005).
Reaction - small molecule participants:
Fe(3+)Cb5 [cytosol]
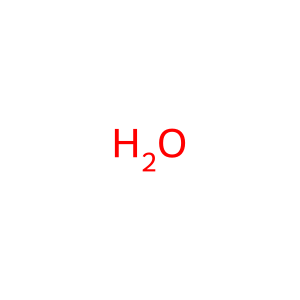
H2O [cytosol]
OLE-CoA [cytosol]
ST-CoA [cytosol]
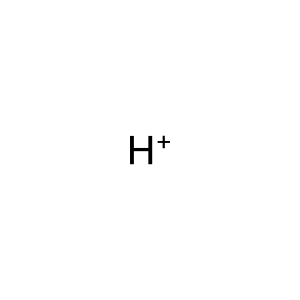
H+ [cytosol]
Fe(2+)Cb5 [cytosol]
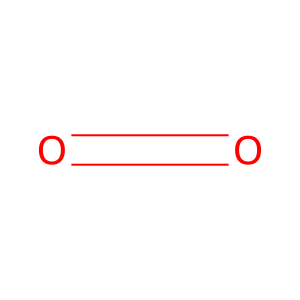
O2 [cytosol]
Reactome.org reaction link: R-HSA-5690565
======
Reaction input - small molecules:
stearoyl-CoA(4-)
hydron
ferrocytochrome b5
dioxygen
Reaction output - small molecules:
ferricytochrome b5
water
oleoyl-CoA
Reactome.org link: R-HSA-5690565