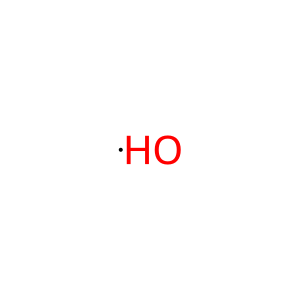
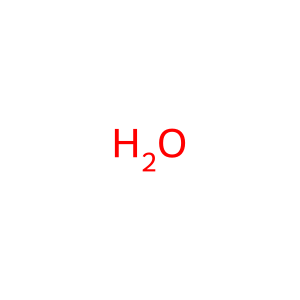
Reaction: Hydroxyl-initiated lipid peroxidation
Membranes are formed by amphiphilic lipids which in most cases studied are glycerophospholipids, composed of two fatty acids, a glycerol moiety, a phosphate group and a variable head group. Bacterial membranes present a large diversity of amphiphilic lipids, including phosphatidylglycerol, phosphatidylethanolamine, cardiolipin and the less frequent phospholipids such as phosphatidylcholine and phosphatidylinositol. Bacteria can also form phosphorus-free membrane lipids such as ornithine lipids, sulfolipids, diacylglyceryl-N,N,N-trimethylhomoserine, glycolipids, diacylglycerol, hopanoids and others. Commonly, the hydrophobic moieties of amphiphilic membrane lipids are formed by linear fatty acids that can be saturated or unsaturated (containing often one and rarely two or more double bonds). (OH.)-dependent abstraction of a hydrogen atom from an unsaturated fatty acid initiates the process of lipid peroxidation by generating a lipid radical, which rapidly adds oxygen to form a lipid peroxyl radical LOO. (not shown here). The peroxyl radicals in turn can further react with lipid molecules to continue the chain reaction, producing lipid hydroperoxides (LOOH), that can break down to more radical species