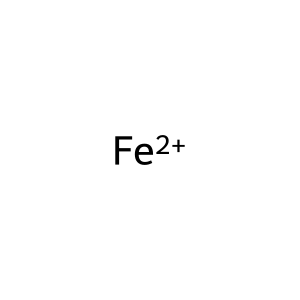Reaction: Superoxide anion reacts with Fe-S cluster
- in pathway: ROS and RNS production in phagocytes
Iron-sulfur (Fe-S) clusters are ubiquitous, evolutionary ancient and functionally versatile prosthetic groups found in a variety of metalloproteins. In most Fe-S proteins, the clusters function as electron-transfer groups in mediating one-electron redox processes. Fe-S clusters may also participate in iron/sulfur storage or regulate enzyme activity and substrate binding. As stress sensors, Fe-S clusters may regulate gene expression. Fe-S clusters have variable compositions such as 2Fe-2S, 3Fe-4S, 4Fe-4S centers. Solvent-exposed [4Fe-4S](2+) clusters are sensitive to oxidation and can be damaged (or disassembled) by reactive oxygen species. Superoxide (O2.-) and hydrogen peroxide (H2O2) oxidize [4Fe-4S](2+) into unstable [4Fe-4S](3+) intermediate, which is degraded to a [3Fe-4S](+) cluster. This process releases free iron (Fe(2+)) and inactivates the enzyme. High concentration of Fe(2+) under oxidative stress elevates ROS toxicity by catalyzing Fenton reaction that generates hydroxyl radical (OH.) from H2O2. Hydroxyl radical reacts with all macromolecules, including proteins, peptidoglycans, lipids or DNA.
Reaction - small molecule participants:
Fe2+ [cytosol]
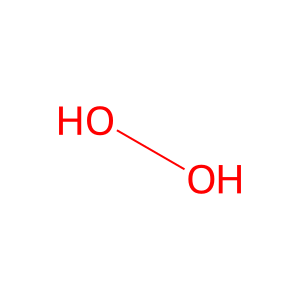
H2O2 [cytoplasm]
O2- [cytoplasm]
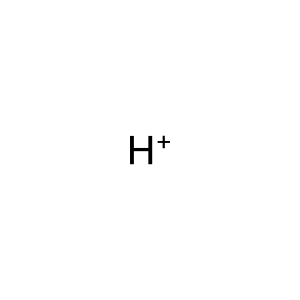
H+ [cytoplasm]
Reactome.org reaction link: R-HSA-6789109
======
Reaction input - small molecules:
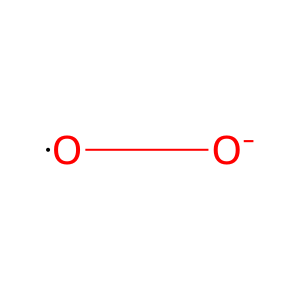
superoxide
hydron
Reaction output - small molecules:
iron(2+)
hydrogen peroxide
Reactome.org link: R-HSA-6789109