Reaction: ASS1 tetramer:NMRAL1 dimer:NADPH transforms L-Asp and L-Cit to ARSUA
- in pathway: Urea cycle
Cytosolic argininosuccinate synthase (ASS1 tetramer) catalyzes the reaction of aspartate (L-Asp), citrulline (L-Cit), and ATP to form argininosuccinate (ARSUA), AMP, and pyrophosphate (PPi). The function of the human enzyme in vivo is inferred from the hypercitrullinemia observed in patients with defective forms of the enzyme (e.g., Engel et al. 2009). The enzyme is active as a homotetramer (O'Brien 1980; Karlberg et al. 2008) and binds NmrA-like family domain-containing protein 1 (NMRAL1). NMRAL1 is a redox sensor protein that can undergo restructuring and subcellular redistribution in response to changes in intracellular NADPH/NADP+ levels. Under normal NADPH levels, it can form an asymmetrical dimer with one subunit occupied by one NADPH molecule, hiding the binding site for ASS1 thus impairing its activity and reducing the production of nitric oxide (Zheng et al. 2007, Zhao et al. 2008).
Reaction - small molecule participants:
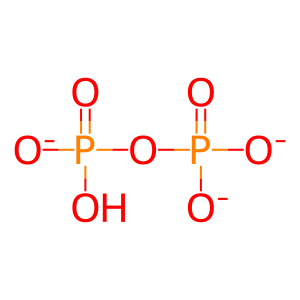
PPi [cytosol]
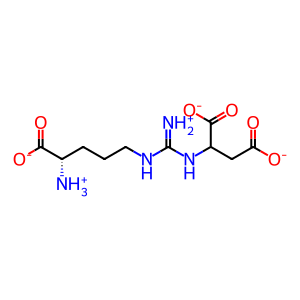
ARSUA [cytosol]
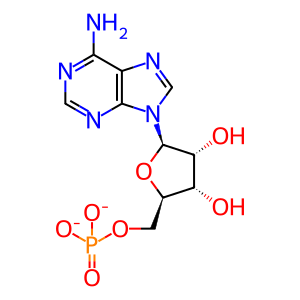
AMP [cytosol]
ATP [cytosol]
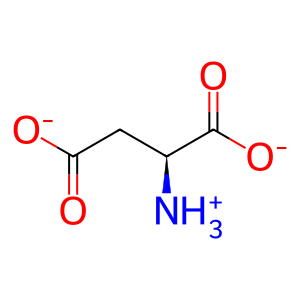
L-Asp [cytosol]
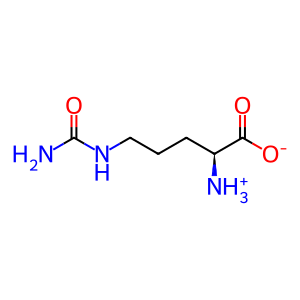
L-Cit [cytosol]
Reactome.org reaction link: R-HSA-70577
======
Reaction input - small molecules:
ATP(4-)
L-aspartate(1-)
L-citrulline zwitterion
Reaction output - small molecules:
diphosphate(3-)
(N(omega)-L-arginino)succinate(1-)
adenosine 5'-monophosphate(2-)
Reactome.org link: R-HSA-70577