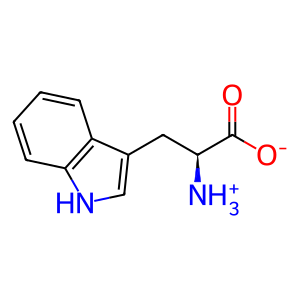
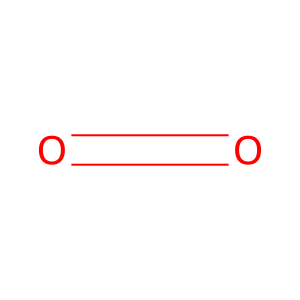
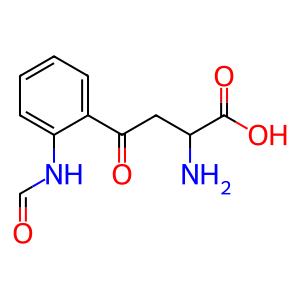
Reaction: TDO tetramer dioxygenates L-Trp to NFK
- in pathway: Tryptophan catabolism
Cytosolic tryptophan 2,3-dioxygenase (TDO) tetramer catalyzes the conversion of L-tryptophan and oxygen to formylkynurenine. The structure and catalytic properties of the human enzyme are inferred from the close similarity of its predicted amino acid sequence (Comings et al. 1995) to that of the well-studied rat enzyme (Dick et al. 2001). In the body, TDO is found predominantly in the liver and is induced by metabolites such as tryptophan and histidine, and by glucocorticoids. These properties, together with TDO's narrow substrate specificity, are consistent with the hypothesis that the enzyme functions functions primarily in tryptophan catabolism and NAD biosynthesis (Taylor and Feng 1991).
Reaction - small molecule participants:
NFK [cytosol]
L-Trp [cytosol]
O2 [cytosol]
Reactome.org reaction link: R-HSA-71188
======
Reaction input - small molecules:
L-tryptophan zwitterion
dioxygen
Reaction output - small molecules:
N-formylkynurenine
Reactome.org link: R-HSA-71188