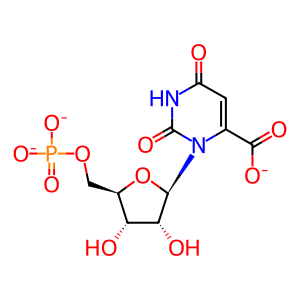
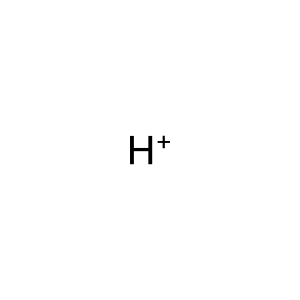
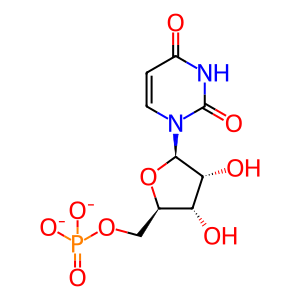
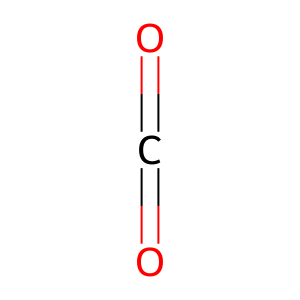
Reaction: UMPS dimer decarboxylates OMP to UMP
- in pathway: Pyrimidine biosynthesis
The decarboxylation of orotidine 5'-monophosphate (OMP) to form uridine 5'-monophosphate (UMP) is catalyzed by the orotidine 5'-phosphate decarboxylase activity of the bifunctional "uridine monophosphate synthetase (orotate phosphoribosyl transferase and orotidine 5'-decarboxylase)" protein. While purified human protein has not been characterized in detail, the close similarity of the human gene to that encoding the well-studied hamster protein, and the demonstration that mutations in the human gene are associated with failure to convert orotate to UMP in vivo, provide convincing evidence that the human uridine monophosphate synthetase protein indeed catalyzes these two reactions (McClard et al. 1980; Suchi et al. 1997). The active form of the human protein is a dimer (Yablonski et al. 1996; Wittmann et al. 2008).
Reaction - small molecule participants:
UMP [cytosol]
CO2 [cytosol]
OMP [cytosol]
H+ [cytosol]
Reactome.org reaction link: R-HSA-73564
======
Reaction input - small molecules:
orotidine 5'-phosphate(3-)
hydron
Reaction output - small molecules:
uridine 5'-monophosphate(2-)
carbon dioxide
Reactome.org link: R-HSA-73564