Reaction: N-atom dealkylation of caffeine
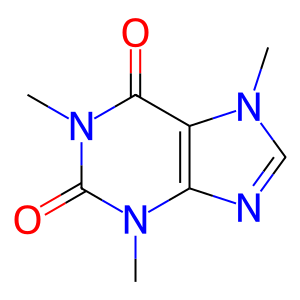
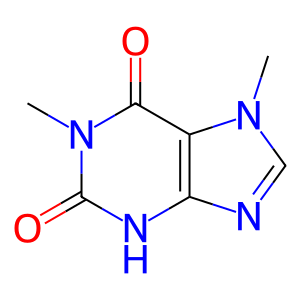
Caffeine is one of the world's most frequently consumed xenobiotic. The major source of caffeine comes from tea and coffee. Caffeine is extensively metabolized in humans with at least 17 metabolites formed in its biotransformation. CYP1A2 is a prominent enzyme in the formation of an important metabolite of caffeine (paraxanthine) by N3-demethylation.
Reaction - small molecule participants:
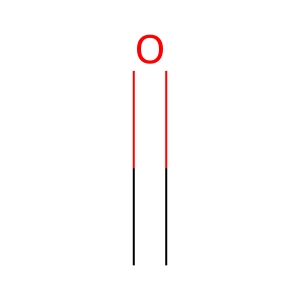
CH2O [endoplasmic reticulum lumen]
H2O [endoplasmic reticulum lumen]
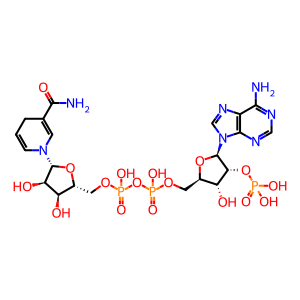
NADP+ [endoplasmic reticulum lumen]
Paraxanthine [endoplasmic reticulum lumen]
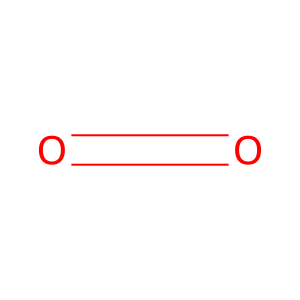
O2 [endoplasmic reticulum lumen]
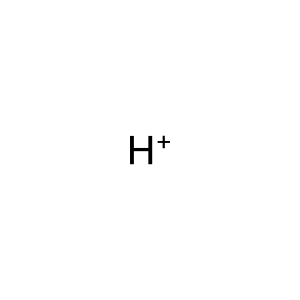
H+ [endoplasmic reticulum lumen]
CAF [endoplasmic reticulum lumen]
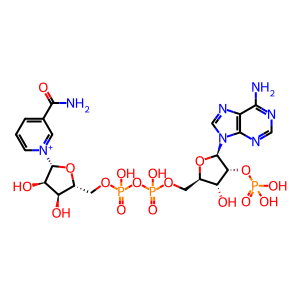
NADPH [endoplasmic reticulum lumen]
Reactome.org reaction link: R-HSA-76426
======
Reaction input - small molecules:
dioxygen
hydron
caffeine
NADPH
Reaction output - small molecules:
formaldehyde
water
NADP(+)
1,7-dimethylxanthine
Reactome.org link: R-HSA-76426