Reaction: Tyrosine kinases phosphorylate the receptor
- in pathway: Interleukin-3, Interleukin-5 and GM-CSF signaling
Phosphorylation of the receptor common beta chain (Bc) creates binding sites for proteins that trigger subsequent signaling cascades (Pawson & Scott, 1997). The cytoplasmic region of Bc contains several tyrosines that become phosphorylated on cytokine binding (Sorensen et al. 1989, Duronio et al. 1992, Sakamaki et al. 1992, Pratt et al. 1996). One site is Y766 (numbered as Y750 by Sakamaki et al. 1992 and many other publications). Phosphorylation of Bc in response to GM-CSF/IL3 is observed at low temperatures (4 degrees C) that prevent the phosphorylation of other proteins, suggesting that the kinase responsible is likely to be physically associated with the receptor complex prior to stimulation (Miyajima et al. 1993). JAK2 is activated in response to IL-3, IL-5 and GM-CSF but signaling via JAK/STAT is not dependent on Bc tyrosine phosphorylation (Okuda et al. 1997). Based on these observations and the role of JAK1/3 in IL-2 signaling, JAK2 is believed to be the most likely candidate responsible for the phosphorylation of Bc (Guthridge et al. 1998). To represent the possible phosphorylation of Bc by kinases other than JAK2, this reaction includes receptor complexes with both active and inactive JAK2. Phosphorylation is represented only where this is necesssary for subsequent signaling; phosphorylation at other positions is probable.
Reaction - small molecule participants:
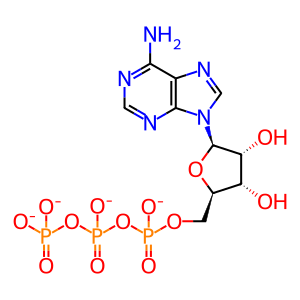
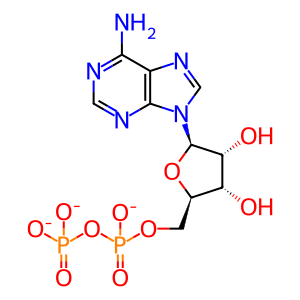
ADP [cytosol]
ATP [cytosol]
Reactome.org reaction link: R-HSA-879907
======
Reaction input - small molecules:
ATP(4-)
Reaction output - small molecules:
ADP(3-)
Reactome.org link: R-HSA-879907