Reaction: Lactoperoxidase (LPO) produces OSCN-
Lactoperoxidase (LPO, also known as salivary peroxidase SPO) is a member of heme-containing peroxidase (XPO) family (Furtmüller PG et al. 2006). LPO has been identified as an antimicrobial agent within exocrine gland secretions such as milk, saliva, and tears through the oxidation of thiocyanate ion (SCN-) by hydrogen peroxide (H2O2) to yield the intermediary oxidation product hypothiocyanite ion (OSCN-), which possesses broad-spectrum of antimicrobial activity (Pruitt KM et al. 1988; Thomas EL et al. 1994; Shin K et al. 2000; Wijkstrom-Frei C et al. 2003; Tahboub YR et al. 2005; Ihalin R et al. 2006; Ashby MT 2008; Welk A et al. 2009, 2011; Bafort F et al. 2014). (OSCN-) oxidises sulphydryls of essential proteins of a microorganism, resulting in an alteration in its cellular functions (Hoogendoorn H et al. 1977; Thomas EL & Aune TM 1978; Mickelson MN 1979; Hawkins CL 2009). Functional alterations of microorganisms cause their growth inhibition and/or death. Structural studies showed that mammalian LPO functions as a monomeric single polypeptide chain which is linked to heme in the catalytic site (Singh AK et al. 2008; Sharma S et al. 2013). The dual oxidases DUOX1 and DUOX2 are the H2O2-producing isoforms of the NADPH oxidase family found in epithelial cells are thought to support LPO-mediated killing of invading pathogens (Forteza R et al. 2005; Fischer H 2009). In healthy individuals, (SCN-) is thought to originate primarily from the diet.
All the members of XPO family catalyze a similar multi-step reaction by oxidizing the heme iron in the catalitic site from Fe(III) to Fe(IV)=O and a porphyrin or aromatic side chain to a cationic radical (Furtmüller PG et al. 2006; Davies MJ et al. 2008; Gumiero A et al. 2011; Bafort F et al. 2014). The classic peroxidases catalytic cycle begins in the presence of H2O2 which reacts rapidly and reversibly with the native state of peroxidases (enzyme:Fe(III) state). Two electrons transfer from the native enzyme:Fe(III) to H2O2 generates a ferryl pi-cation-radical (E-Fe(IV)=O.+pi) intermediate named Compound I and reduces H2O2 into water. In the presence of a halogen (Cl-, Br-, or I-) or a pseudohalogen (SCN-), Compound I is reduced back to its native enzymatic form through a two-electron transfer while the (pseudo)halogen is oxidized into a hypo(pseudo)halide. Hypo(pseudo)halides are powerful oxidants with antimicrobial activity. Alternatively, Compound I is also capable of oxidizing multiple organic and inorganic molecules (AH2) by two successive sequential one-electron-transitions generating their corresponding radicals (.AH) and the peroxidase intermediate Compound II (enzyme:Fe(IV)=O) and the native enzyme:Fe(III), respectively (Furtmüller PG et al. 2006; Zederbauer M et al. 2007; Davies MJ et al. 2008; Gumiero A et al. 2011; Bafort F et al. 2014).
The Reactome event describes the halogenation cycle where LPO-derived Compound I catalyzes the oxidation of thiocyanate ion (SCN-) to hypothiocyanite ion (OSCN-).
Reaction - small molecule participants:
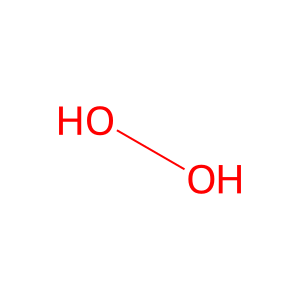
H2O [extracellular region]
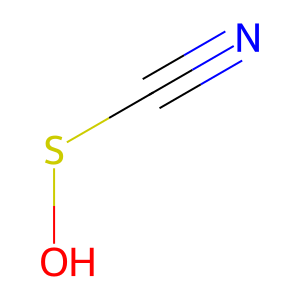
OSCN- [extracellular region]
H2O2 [extracellular region]
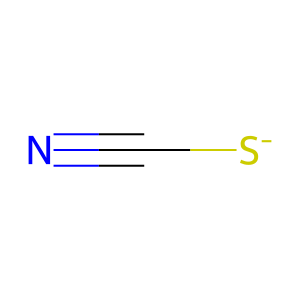
SCN(-) [extracellular region]
======
Reaction input - small molecules:
hydrogen peroxide
thiocyanate
Reaction output - small molecules:
water
hypothiocyanous acid