Reaction: STARD10 binds LPCAT1 and PC
- in pathway: Synthesis of PC
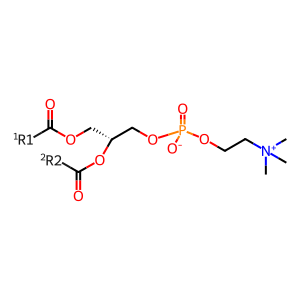
The steroidogenic acute regulatory (StAR) protein-related lipid transfer (START) domain proteins constitute a family of evolutionarily conserved and widely expressed proteins that have been implicated in lipid transport, metabolism, and signaling. Human PCTP-like protein (STARD10) (Olayioye et al. 2005) is thought to be a dual specificity lipid transfer protein capable of shuttling phosphatidylcholine (PC) (and phosphatidylethanolamine (PE), not shown here) between intracellular membranes, especially to lamellar body membranes. Saturated PC is a major component of pulmonary surfactant, a mixture of proteins and phospholipids that plays an important role in facilitating gas exchange by maintaining alveolar stability. After synthesis in the endoplasmic reticulum, saturated PC is transported to lamellar bodies (LBs) for storage prior to secretion. Lysophosphatidylcholine acyltransferase 1 (LPCAT1) mediated reacylation is a final step in saturated PC synthesis prior to transport and LPCAT1 is proposed to form a complex with STARD10 at the ER membrane to facilitate the synthesis then transport of saturated PC to LBs (Lin et al. 2015).
Reaction - small molecule participants:
PC [endoplasmic reticulum membrane]
Reactome.org reaction link: R-HSA-8873923
======
Reaction input - small molecules:
1,2-diacyl-sn-glycero-3-phosphocholine
Reaction output - small molecules:
Reactome.org link: R-HSA-8873923