Reaction: B3GALNT2 transfers GalNAc to GlcNAc-Man-DAG1
- in pathway: O-linked glycosylation
Three enzymes are involved in the biosynthesis of a phosphorylated O-mannosyl trisaccharide structure (N-acetylgalactosamine-beta-1,3-N-acetylglucosamine-beta-1,4-(phosphate-6-)mannose) present in alpha-dystroglycan (DAG1), which is required for binding laminin G-like domain-containing extracellular proteins with high affinity. Defects in any of these enzymes can lead to congenital muscular dystrophy.
The second step is catalysed by UDP-GalNAc:beta-1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2), an ER membrane-associated enzyme that transfers N-acetylgalactosamine (GalNAc) to GlcNAc-Man-DAG1 via a 1-3 glycosidic bond (Hiruma et al. 2004, Yoshida-Moriguchi et al. 2013). Defects in B3GALNT2 can cause muscular dystrophy-dystroglycanopathy congenital with brain and eye anomalies A11 (MDDGA11), a hypoglycosylation defect resulting in a reduced ability of DAG1 to bind laminin and other extracellular matrix ligands. The disorder is characterised by dystroglycanopathy with muscle and brain anomolies (Stevens et al. 2013).
The second step is catalysed by UDP-GalNAc:beta-1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2), an ER membrane-associated enzyme that transfers N-acetylgalactosamine (GalNAc) to GlcNAc-Man-DAG1 via a 1-3 glycosidic bond (Hiruma et al. 2004, Yoshida-Moriguchi et al. 2013). Defects in B3GALNT2 can cause muscular dystrophy-dystroglycanopathy congenital with brain and eye anomalies A11 (MDDGA11), a hypoglycosylation defect resulting in a reduced ability of DAG1 to bind laminin and other extracellular matrix ligands. The disorder is characterised by dystroglycanopathy with muscle and brain anomolies (Stevens et al. 2013).
Reaction - small molecule participants:
UDP [endoplasmic reticulum lumen]
UDP-GalNAc [endoplasmic reticulum lumen]
Reactome.org reaction link: R-HSA-8931648
======
Reaction input - small molecules:
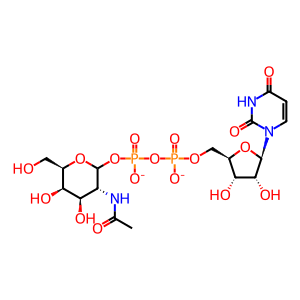
UDP-N-acetyl-D-galactosamine(2-)
Reaction output - small molecules:
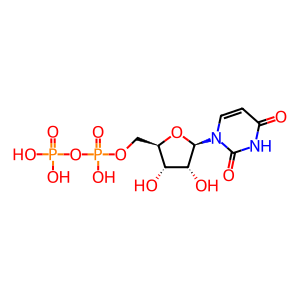
UDP
Reactome.org link: R-HSA-8931648