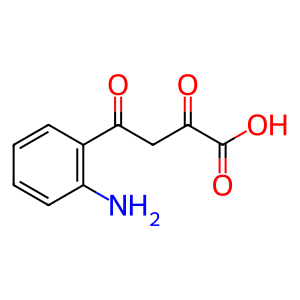
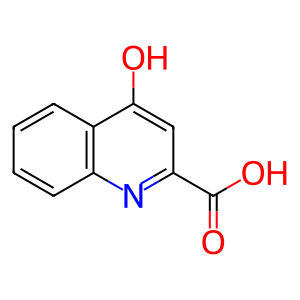
Reaction: 4-(2-aminophenyl)-2,4-dioxobutanoate => kynurenic acid + H2O [mitochondrial]
Biochemical studies of kynurenine transamination in vitro invariably measure kynurenic acid, not 4-(2-aminophenyl)-2,4-dioxobutanoate, the expected transamination product. The reaction annotated here has not been demonstrated directly. As noted by Miller et al. (1953), "The keto acid assumed to be formed prior to ring closure in the conversion of kynurenine to kynurenic acid has not yet been detected. In principle, such detection should be possible, since it is sufficiently stable to have been synthesized. It also remains to be established whether ring closure is spontaneous, enzymatic, or both. The formation of kynurenic acid from L-kynurenine by the L-amino acid oxidase of Neurospora suggests, however, that ring closure can be spontaneous, unless the somewhat improbable assumption is made that Neurospora filtrate contained the ring-closing enzyme."