Reaction: CES3 hydrolyses CHEST to CHOL and LCFA(-)
- in pathway: LDL clearance
In macrophage foam cells, the hydrolysis of cholesteryl esters (CHESTs) is the rate-limiting step in the removal of free cholesterol (CHOL) from these cells. CHOL is transported via transport vesicles and can be used for cellular functions or removed from the cell by ABCA1 to create new HDL particles. Accumulation of CHESTs in macrophage foam cells is key to atherosclerotic plaque formation and occurs as a result of an imbalance between CHOL influx and efflux pathways. The main hydrolase that hydrolyses CE in macrophages is neutral cholesterol ester hydrolase 1 (NCEH1). Carboxylesterases (CESs), usually involved in the hydrolysis of drugs, can also hydrolyse CHESTs with CES1 responsible for >70% of the total CES hydrolytic activity in macrophages, thus playing an important antiatherogenic role. CES1 knockdown studies reveal a compensatory increase in the expression of CES3, expressed at <30% of the level of CES1 in human macrophages, which restores intracellular CHEST hydrolytic activity and CHOL efflux (Zhao et al. 2012). Human CES3 isoproteins are predicted to be either secreted or retained in the cytosol (Holmes et al. 2010) but the exact location is currently unknown.
Reaction - small molecule participants:
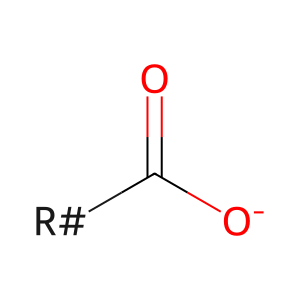
LCFA(-) [cytosol]
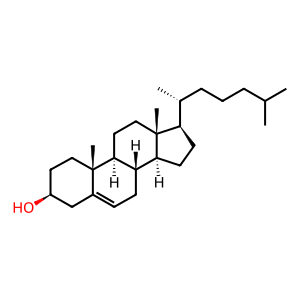
CHOL [transport vesicle membrane]
H2O [cytosol]
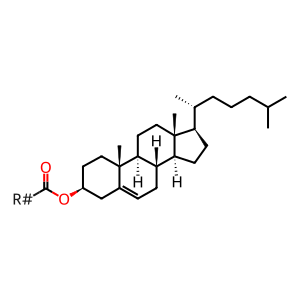
CHEST [lipid droplet]
LCFA(-) [cytosol]
CHOL [transport vesicle membrane]
H2O [cytosol]
CHEST [lipid droplet]
Reactome.org reaction link: R-HSA-8937442
======
Reaction input - small molecules:
water
cholesteryl ester
water
cholesteryl ester
Reaction output - small molecules:
long-chain fatty acid anion
cholesterol
long-chain fatty acid anion
cholesterol
Reactome.org link: R-HSA-8937442