Reaction: HSP90-dependent ATP hydrolysis promotes release of ESR:ESTG from chaperone complex
- in pathway: ESR-mediated signaling
Release of the estrogen receptor from the chaperone complex requires requires HSP90-dependent ATP hydrolysis, and occurs at the same rate in the presence and absence of ligand (Smith et al, 1992; Smith et al, 1993; Aumais et al, 1997; Grenert et al, 1997; Obermann et al, 1998; Panaretou et al, 1998; reviewed in Smith and Toft, 2008). In the absence of ligand, released ERs are recaptured by HSP40 through its interaction with the ligand binding domain (LBD), priming reassembly of the chaperone complex. Ligand binding may result in the loss of the HSP40-binding site, allowing the receptor to escape repetitive rounds of chaperone complex assembly, and freeing it for DNA-binding (reviewed in Smith and Toft, 2008).
Reaction - small molecule participants:
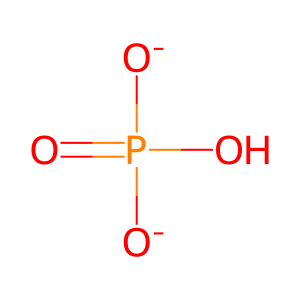
Pi [nucleoplasm]
ADP [nucleoplasm]
Reactome.org reaction link: R-HSA-8939203
======
Reaction input - small molecules:
Reaction output - small molecules:
hydrogenphosphate
ADP(3-)
Reactome.org link: R-HSA-8939203