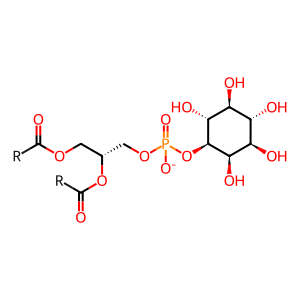
Reaction: GPLD1 hydrolyses GPI-anchors from proteins
Some proteins function at the cell's surface, attached to the plasma membrane via GPI (glycosylphosphatidylinositol) anchors and include enzymes, receptors, cell adhesion molecules and antigens. These GPI-anchored proteins participate in many important cellular functions including immune recognition, complement regulation and intracellular signaling. Phosphatidylinositol-glycan-specific phospholipase D (GPLD1) (Schofield et al. 2000) is a secreted protein that specifically cleaves GPI-anchored proteins by cleaving the linkage between the phosphate and inositol in GPI (Davitz et al. 1987, Low & Prasad 1988). In addition, it also localises to the ER where it can cleave GPI anchor intermediates transiting to the plasma membrane (not shown here). GPLD1 may play a role in the regulation of GPI-anchored proteins on (intra)cellular membranes.
Reaction - small molecule participants:
PI [extracellular region]
H2O [extracellular region]
Reactome.org reaction link: R-HSA-8940388
======
Reaction input - small molecules:
water
Reaction output - small molecules:
1-phosphatidyl-1D-myo-inositol(1-)
Reactome.org link: R-HSA-8940388