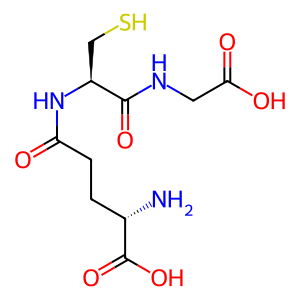
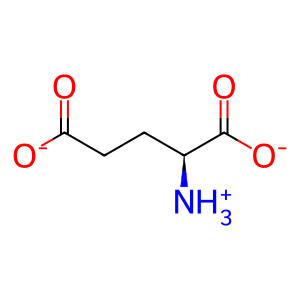
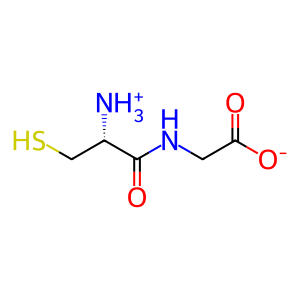
Reaction: GGT dimers hydrolyse GSH
- in pathway: Glutathione synthesis and recycling
GGT (gamma-glutamyl transpeptidase) dimers associated with the plasma membrane (Hanigan & Frierson 1996) hydrolyze extracellular glutathione (GSH) to form cysteinylglycine (CysGly) and glutamate (L-Glu). GGT1 has been extensively characterized. The active dimeric form of the enzyme is generated by autohydrolysis (West et al. 2011) and in vitro can catalyze both the reaction of GSH with water annotated here, and the reaction of GSH with a free amino acid or dipeptide to generate a gamma-glutamyl-amino acid and cysteinylglycine (Castonguay et al. 2007; Pawlak et al. 1989; Tate & Ross 1977; Thompson & Meister 1976). Based on amino acid sequence similarity, Heisterkamp et al. (2008) identified five additional dimeric proteins, GGT2, 3P, 5, 6, and 7, likely to catalyze the same reactions. West et al. (2013), however, found that GGT2 had no catalytic activity in vitro.
Reaction - small molecule participants:
L-Glu [extracellular region]
CysGly [extracellular region]
H2O [extracellular region]
GSH [extracellular region]
Reactome.org reaction link: R-HSA-8943279
======
Reaction input - small molecules:
water
glutathione
Reaction output - small molecules:
L-glutamate(1-)
L-cysteinylglycine zwitterion
Reactome.org link: R-HSA-8943279