Reaction: NEDD8 covalently binds catalytic cysteine of UBA3:NAE1
- in pathway: Neddylation
NEDD8 is attached via a thioester bond to the catalytic cysteine of its E1 as the first step in its transfer to substrates (Walden et al, 2003). The NEDD8 E1 is a heterodimer consisting of UBA3 and NAE1, and transfers NEDD8 to the E2 enzymes from a 'doubly loaded' state. In the first step, NEDD8 binds to the adenylation site on the NAE1 subunit in conjuction with ATP and Mg2+, generating a covalently modified NEDD8-adenylate conjugate. This conjugation activates the NEDD8 C-terminus for chemical attack by the thiol group of the catalytic cysteine of the UBA3 subunit. Catalysis is likely facilitated by a conformational change in the E1 enzyme. After catalysis, NEDD8 is covalently bound in the E1 catalytic site, leaving the adenylation site free to bind another NEDD8 molecule in the second step, prior to NEDD8 transfer to an E2 enzyme (Walden et al, 2003; Huang et al, 2004; Huang et al, 2005; Huang et al, 2007).
Reaction - small molecule participants:
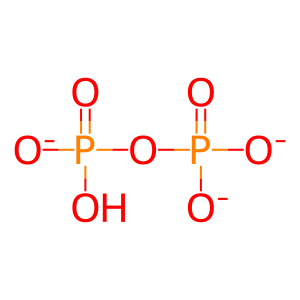
PPi [cytosol]
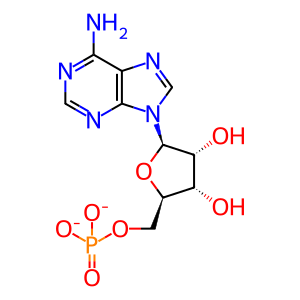
AMP [cytosol]
ATP [cytosol]
Reactome.org reaction link: R-HSA-8951648
======
Reaction input - small molecules:
ATP(4-)
Reaction output - small molecules:
diphosphate(3-)
adenosine 5'-monophosphate(2-)
Reactome.org link: R-HSA-8951648