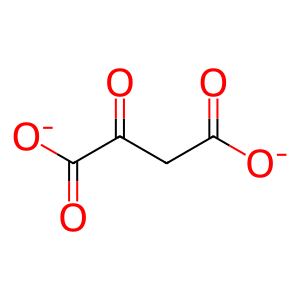
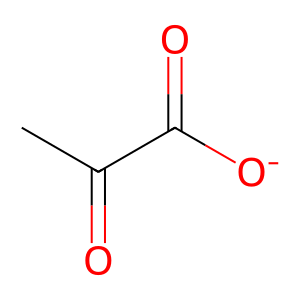
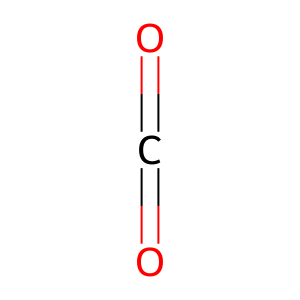
Reaction: FAHD1:Zn2+ dimer hydrolyses OA to PYR
- in pathway: Citric acid cycle (TCA cycle)
Fumarylacetoacetate hydrolase domain containing protein 1 (FAHD1) (Pircher et al. 2011, 2015, Jansen-Duerr et al. 2016) was identified to display a bi-functional catalytic mechanism (Weiss et al. 2018), being able to hydrolyse acylpyruvates (Pircher et al. 2011) similar to fumarylpyruvate hydrolase NagK of Ralstonia sp. (acetylpyruvate: vmax = 0,135 µmol/min/mg, KM = 4,6 µM), and to cleave oxaloacetate (OAA) via decarboxylation (Pircher et al. 2015) (OAA: vmax = 0,21 µmol/min/mg, KM = 32 µM). The enzyme is of dimeric form (Manjasetty et al. 2004) and uses Mg2+ or Mn2+ as cofactor. It is localized in the mitochondrial matrix (Pircher et al. 2011, Trukhina et al. 2002, Di Berardino et al. 1996). Its identification as ODx (Pircher et al. 2015) renders FAHD1 a possible antagonist to pyruvate carboxylase (PC) at a central position in the TCA cycle(Jansen-Duerr et al. 2016). It is believed that the ability of FAHD1 to decarboxylate OAA provides the basis of its requirement for maintaining healthy mitochondria in certain cells and tissues (Taferner et al. 2015, Petit et al. 2017). However, further studies of this topic are warranted.
Reaction - small molecule participants:
PYR [mitochondrial matrix]
CO2 [mitochondrial matrix]
OA [mitochondrial matrix]
Reactome.org reaction link: R-HSA-9012016
======
Reaction input - small molecules:
oxaloacetate(2-)
Reaction output - small molecules:
pyruvate
carbon dioxide
Reactome.org link: R-HSA-9012016