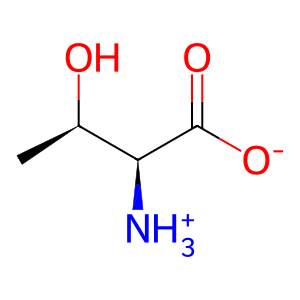
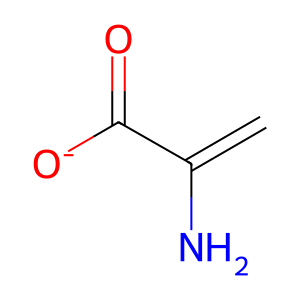
Reaction: SDS dimers:PXLP dehydrate L-Thr to 2AA
- in pathway: Threonine catabolism
Various PXLP-dependent enzymes can catalyse α, β-elimination reactions of amino acid substrates, ultimately yielding α-keto (or 2-oxo-) acid products. However, these enzymes, such as L-serine dehydratase/L-threonine deaminase (SDS aka TDH), only form the enamine intermediate as the remainder of the reaction occurs in solution with the enamine intermediate tautomerising to the imine form, which then spontaneously hydrolyzes to the final α-keto acid product (Downs & Ernst 2015). SDS can dehydrate L-threonine (L-Thr) to form the intermediate enamine 2-aminoacrylate (2AA), which can damage the pyridoxal 5'-phosphate cofactor (PXLP) of various enzymes, causing inactivation and significant cellular damage if allowed to accumulate (Lambrecht et al. 2013). SDS exists as a homodimer and requires PXLP for activity (Sun et al. 2005). An isoform of SDS, serine dehydratase-like (SDSL aka SDH2), is found in human cancer cell lines and possesses lower catalytic activity than SDS (Yamada et al. 2008).
Reaction - small molecule participants:
H2O [cytosol]
2AA [cytosol]
L-Thr [cytosol]
Reactome.org reaction link: R-HSA-9014627
======
Reaction input - small molecules:
L-threonine zwitterion
Reaction output - small molecules:
water
2-aminoacrylate
Reactome.org link: R-HSA-9014627